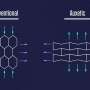
The CSU team instead demonstrates in the paper a way to first break an existing pyrimidine heterocycle and then rebuild it. Credit: Nature (2024). DOI: 10.1038/s41586-024-07474-1
Researchers at Colorado State University have published findings in Nature that could be useful to speed the development of new pharmaceuticals and pesticides.
Professors Andy McNally and Robert Paton led the work through the Department of Chemistry at CSU. The paper outlines an approach for deconstructing and then reassembling common compounds known as heterocycles to achieve new characteristics. These compounds are often a starting point for research in a variety of fields, and scientists are constantly working to modify them to improve or optimize medicines, for example.
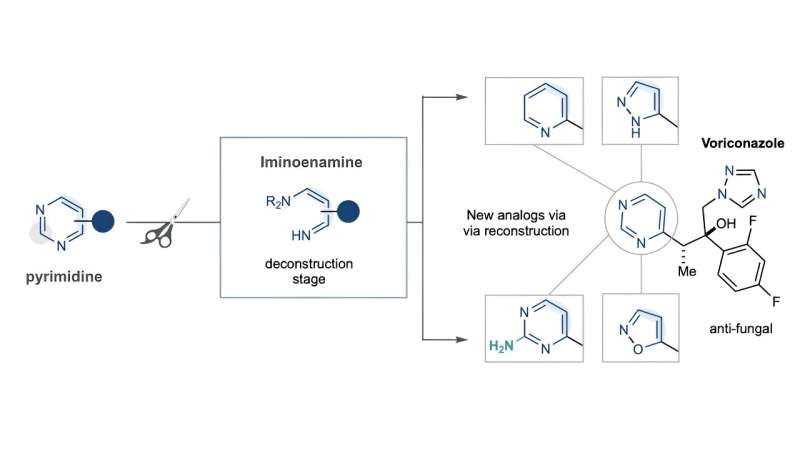
Usually, creating these compounds is done at the start of the development process, with researchers building off known combinations through multiple steps to arrive at a possible drug candidate for testing. The CSU team instead demonstrates in the paper a way to first break an existing pyrimidine heterocycle and then rebuild it to contain nitrogen rather than carbon along the edge of the structures.
That change at the periphery allows for a new set of beneficial chemical reactions that would have been hard or impossible to achieve with these structures before. This approach, McNally said, removes the need to do extensive and time-consuming synthesis from the original simple precursor compound outward. He said this kind of work is known as molecular editing.
"Our approach is counter intuitive but is more efficient," said McNally, who holds the Albert I. Meyers Chair in Organic Chemistry. "By pulling these compounds apart and rebuilding them at the end, we can rapidly change them into slightly different, more potent versions that can then speed development of the final drug without having to work through a lot of potential combinations—some of which may never work out at all."
McNally's team specializes in synthetic organic chemistry, or the science of creating new and needed molecules in a lab. While his team led the experimental portion of the research, they worked closely with Paton's team to develop and use computational methods to test their approaches and to better describe the transition states. Additional authors on the research from both groups in the department include graduate students Louis de Lescure, Benjamin Uhlenbruck and Celena Josephitis.
McNally said the strategy described in the paper can be used in many fields and builds on a tradition of research excellence in synthetic chemistry at CSU. He said his team is now focusing on how this approach can also be used to better understand drug activity in the body ahead of use by the public.
"Any drug that goes on the market must first go through toxicology reports to ensure it doesn't wind up where it doesn't belong and to better quantify its activity in the body," he said.
"Our approach here can be used to increase the mass of these compounds slightly without changing the chemistry. Doing so allows researchers to use mass spectrometry to better detect its path and activity and would again speed development and understanding of these needed chemicals."
More information: Benjamin J. H. Uhlenbruck et al, A deconstruction-reconstruction strategy for pyrimidine diversification, Nature (2024). DOI: 10.1038/s41586-024-07474-1
Journal information: Nature
Provided by Colorado State University