This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Chemists devise easier new method for making a common type of building block for drugs
Ring-shaped chemical structures called saturated heterocycles are found in most FDA-approved drugs but are often difficult to create. Scripps Research chemists have just developed a surprisingly easy method for making many of these sought-after compounds from inexpensive starting chemicals.
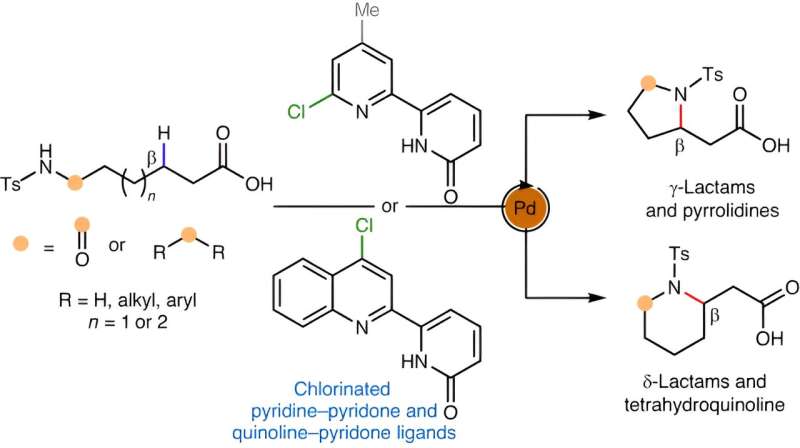
The new method, described in a paper that appears April 11, 2024, in Nature Synthesis, enables chemists to make saturated heterocycles from relatively simple, chain-like amine compounds.
The researchers demonstrated the power of their new method by using it to perform an efficient synthesis of stemoamide, a complex plant-derived compound found in traditional medicines.
"These new reactions should make it easier than ever to construct saturated heterocycles with ring sizes and structures that are relevant for drug development," says study senior author Jin-Quan Yu, Ph.D., Frank and Bertha Hupp Professor of Chemistry and Bristol Myers Squibb Endowed Chair in Chemistry at Scripps Research.
The first author was Sam Chan, Ph.D., a postdoctoral research associate in the Yu lab during the study.
Saturated heterocycles are cyclic organic compounds whose backbone structure contains at least one non-carbon atom. In heterocyclic drug compounds, the non-carbon atom is usually a nitrogen atom, which often plays a crucial role in determining the compound's chemical properties and therapeutic effectiveness. However, current methods for making these much-valued compounds are quite limited. Even when they can be used, they tend to be cumbersome or require relatively expensive and complex starting materials.
"The most convenient way to forge such a ring would be to take a readily available aliphatic amine compound, which contains nitrogen, and stitch that nitrogen onto another part of its carbon backbone, essentially folding the molecule onto itself," Yu says.
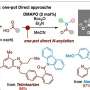
This would involve the removal of a hydrogen atom to allow the new carbon-nitrogen bond to form—making it a type of "C-H activation" reaction, long the specialty of the Yu lab. No such C-H reaction for forming cyclic amines has existed—not a practical one, anyway. For the new study, Yu and his team set out to invent one.
The method they ultimately devised included a palladium catalyst for breaking the C-H bond. It also involved a set of molecules called chlorinated pyridine-pyridones that work as so-called ligands with the proper geometry to promote the new C-N bond formation.
The chemists showcased their new approach by easily making dozens of cyclic amine and related structures, including γ- and δ-lactams, pyrrolidines, and tetrahydroquinolines—all of which would be of interest to pharmaceutical chemists.
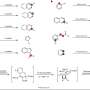
In a final flourish, they showcased the utility of their method with a synthesis—starting almost from scratch with a very simple amine compound—of the plant-derived cyclic amide stemoamide, which has been viewed as a potential starting point for new anti-inflammatory drugs.
Yu and his team are currently working to extend their new approach to make other types of saturated heterocycle.
"Palladium-catalyzed methylene C(sp3)–H lactamization and cycloamination enabled by chlorinated pyridine-pyridone ligands" was co-authored by Hau Sun Sam Chan, Yilin Lu, and Jin-Quan Yu, all of Scripps Research.
More information: Hau Sun Sam Chan et al, Palladium-catalysed methylene C(sp3)–H lactamization and cycloamination enabled by chlorinated pyridine–pyridone ligands, Nature Synthesis (2024). DOI: 10.1038/s44160-024-00517-5
Journal information: Nature Synthesis
Provided by The Scripps Research Institute