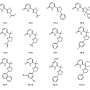
Credit: ACS Applied Materials & Interfaces (2024). DOI: 10.1021/acsami.3c17113
Scientists at NPL worked with Diamond Light Source to publish a study that shows how the chemistry of human cells changes, depending on the structure of their extracellular niche, are major determinants of cell responses and development pathways. The paper is published in ACS Applied Materials & Interfaces.
The human body undergoes renewal by specializing "blank" cells—termed stem cells—into primary cells organized into tissues according to their environment. The environment is created by extracellular matrices or scaffolds on which cells build up tissues and organs.
Cell responses to these matrices provide performance metrics that are critical for the development of cell-based diagnostics and therapies. Some of the important metrics are obtained using microscopy and biological assays, which, however, fail to capture the chemistry of cells and how it changes at different cell-matrix interfaces.
This shortcoming continues to hamper progress in health care and technology innovation, as chemistry is the direct reflection of cellular processes responsible for tissue growth and repair.
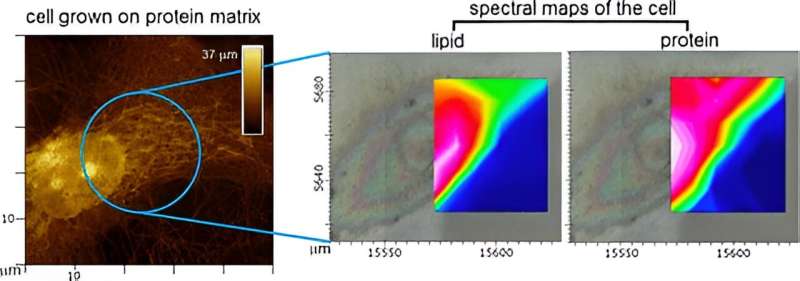
Scientists at NPL set out to address this gap by recording InfraRed maps of human primary and stem cells grown on native and synthetic matrices.
InfraRed spectroscopy can access virtually all chemical information available in the cell but cannot tell the cell from its matrix or recognize different parts of the same cell. Therefore, a correlative approach involving the use of physical imaging, provided by light and atomic force microscopy, to guide chemical spectra was necessary.
To achieve this, NPL collaborated with beamline scientists at Diamond Light Source, biologists from Sheffield and London Colleges and data expertise from Cambridge. Together, they developed a spectral imaging approach which not only allowed them to obtain chemical maps of single cells but also to cross-compare their chemistry signatures in response to matrices exhibiting distinct physical properties.
Their study also demonstrates the efficiency of correlative measurements to explain cell behavior at cell-matrix interfaces in 2D, as well as the need to develop analogous and more advanced methodologies to measure cell-matrix interfaces in 3D, pathing a way to impact for health care and solutions towards human tissue regeneration.
Max Ryadnov, NPL Fellow, said, "The study was an exciting collaboration which provided us with important insights into how the chemical make-up of human cells correlates with the physical changes of the molecular scaffolds that support their growth and development. The study also informed next steps in our developments focusing on correlative measurements of living biological systems."
More information: Emiliana De Santis et al, Hyperspectral Mapping of Human Primary and Stem Cells at Cell–Matrix Interfaces, ACS Applied Materials & Interfaces (2024). DOI: 10.1021/acsami.3c17113
Journal information: ACS Applied Materials and Interfaces
Provided by National Physical Laboratory