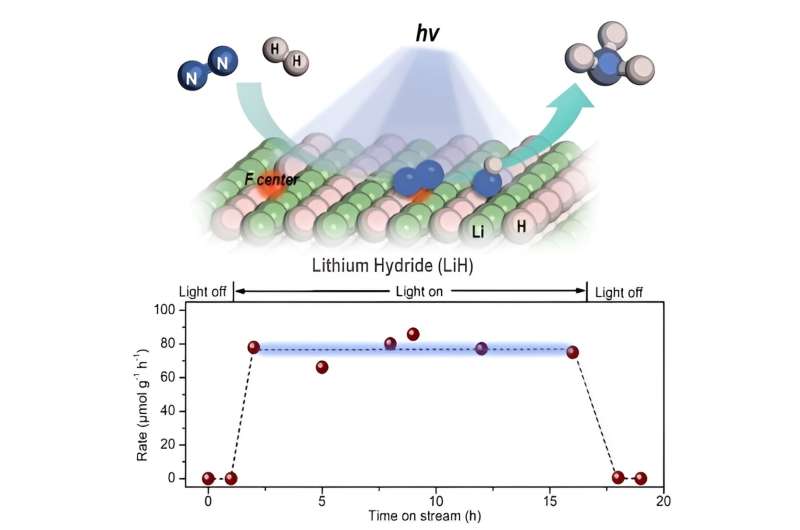
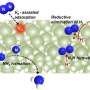
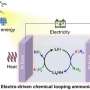
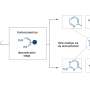
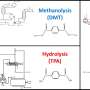
Schematics for the photo-driven nitrogen fixation process of LiH and the photocatalytic ammonia synthesis performance. Credit: Guan Yeqin
Ammonia is essential for food and future energy supply. In the industry, it is mainly produced by the Haber-Bosch process, which operates at high temperatures and pressures. Due to the high energy consumption and carbon emissions of ammonia industry, it is important to develop alternative materials and approaches for efficient N2 reduction to ammonia driven by renewable energy.
A research group led by Prof. Chen Ping from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has realized photo-driven nitrogen fixation and ammonia synthesis mediated by lithium hydride (LiH). The study is published in Nature Chemistry.
LiH is the simplest saline hydride with a band gap of 3.7 eV. It has been investigated for hydrogen storage due to its high hydrogen content (12.5 wt%). However, the dehydrogenation of LiH is thermodynamically unfavorable.
In this study, the researchers found that ultraviolet (UV) illumination of LiH could induce a notable color change from white to light blue, accompanied by the release of a small amount of H2 under ambient conditions. Such a phenomenon implied that under UV illumination, LiH underwent photolysis resulting in photon-generated electrons trapped in its hydrogen vacancy as long-lived and electron-rich F centers, which showed a fundamentally different mechanism for charge carrier separation.
The researchers indicated that illuminated LiH had an electron-rich surface with hydrogen vacancies, which facilitated the activation of N2 to form N-H bond. They co-fed a N2/H2 mixture with a low H2 partial pressure into the LiH powders, leading to photo-catalytic ammonia production under ambient conditions.
"This photochemical route is flexible in operation, which may be amenable to the small-scale and distributed ammonia synthesis powered by intermittent solar energy," said Prof. Chen.
More information: Yeqin Guan et al, Light-driven ammonia synthesis under mild conditions using lithium hydride, Nature Chemistry (2024). DOI: 10.1038/s41557-023-01395-8
Journal information: Nature Chemistry
Provided by Chinese Academy of Sciences