This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers propose electrodriven chemical looping ammonia synthesis mediated by lithium imide
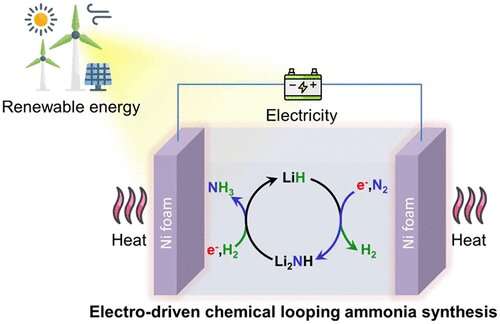
Ammonia (NH3) is a promising energy vector for the storage and utilization of renewable energies. Artificially synthesizing NH3 from its elements requires harsh reaction conditions (400-500 °C, 10–30 MPa) because N2 is inert and nonpolar with a strong N≡N bond. The synthesis of NH3 under mild conditions is still challenging.
Recently, Assoc. Profs. Cao Hujun, Gao Wenbo and their collaborators from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) have proposed a new process for ammonia synthesis using Li2NH as an N carrier via the method of electrodriven chemical looping.
This study was published in ACS Energy Letters.
The researchers carried out this electrodriven chemical looping ammonia synthesis (ECLAS) in a LiCl-NaCl-KCl eutectic electrolytic cell using a nickel foam as electrode.
Electric energy input not only improved the hydrogenation rate of Li2NH, but also promoted the nitrogen fixation reaction of LiH. In addition, the average ammonia production rate of this ECLAS process was nearly eight times higher than that of the thermal-driven CLAS process.
They found that the process contained two electrochemical reactions, one was the nitridation of LiH to form Li2NH, and the other was the hydrogenation of Li2NH to produce ammonia and regenerate LiH. This was different from the reported Li3N-mediated electrochemical ammonia synthesis process, which included three-step reactions: Li ion was reduced to Li, Li fixed dinitrogen to form Li3N, and Li3N was then protonated to produce ammonia and Li+.
"This ECLAS process has a low theoretical operating voltage than the Li3N-mediated electrochemical ammonia synthesis process, and it could work under as low voltages as 2.0 V," said Cao.
More information: Sheng Feng et al, Electrodriven Chemical Looping Ammonia Synthesis Mediated by Lithium Imide, ACS Energy Letters (2023). DOI: 10.1021/acsenergylett.2c02730
Journal information: ACS Energy Letters
Provided by Chinese Academy of Sciences