Transition-metal–free barium hydride mediates dinitrogen fixation and ammonia synthesis
Ammonia is crucial for the manufacture of nitrogen fertilizers. Due to the high energy consumption of industrial ammonia production, the development of alternative materials and approaches for efficient N2 reduction to ammonia under mild conditions is a long-sought goal.
Recently, a research group led by Prof. Chen Ping and Prof. Guo Jianping from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, in collaboration with Prof. Tejs Vegge from Technical University of Denmark, synthesized ammonia via a chemical looping process mediated by a transition-metal-free barium hydride (BaH2) and revealed its mechanism.
This study was published in Angewandte Chemie International Edition on August 2.
Alkali or alkaline earth metal hydrides can fix N2, forming corresponding metal imides and H2. The metal imides then undergo hydrogenation to NH3 and metal hydrides. However, the reaction mechanisms of N2 activation, H2 release and NH3 formation over alkali hydrides are still unclear.
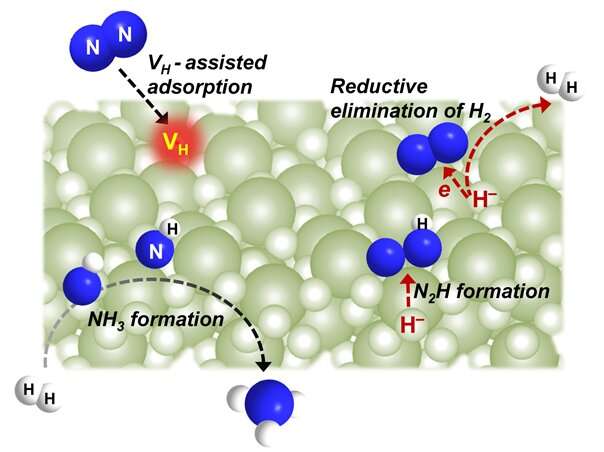
The researchers indicated that the creation of hydrogen vacancies played an important role in N2 fixation process mediated by BaH2.
The creation of hydrogen vacancies led to the formation of multiple coordinatively unsaturated Ba sites, which were responsible for the adsorption and activation of N2. The hydridic hydrogen acted as an electron donor and facilitated the activation of N2 with concurrent H2 release.
They found that the process functionally resembled the molecular hydrido complexes and FeMo cofactor in nitrogenase. Both the hydridic hydrogen and gaseous H2 were involved in the NH3 formation process.
"This is a helpful model for understanding the activation and hydrogenation of N2 to NH3 mediated by alkali and alkaline earth metal hydrides, which is promising in future technologies for nitrogen fixation using transition-metal-free materials," said Prof. Chen.
More information: Yeqin Guan et al, Transition‐Metal‐Free Barium Hydride Mediates Dinitrogen Fixation and Ammonia Synthesis, Angewandte Chemie International Edition (2022). DOI: 10.1002/anie.202205805
Journal information: Angewandte Chemie International Edition
Provided by Chinese Academy of Sciences