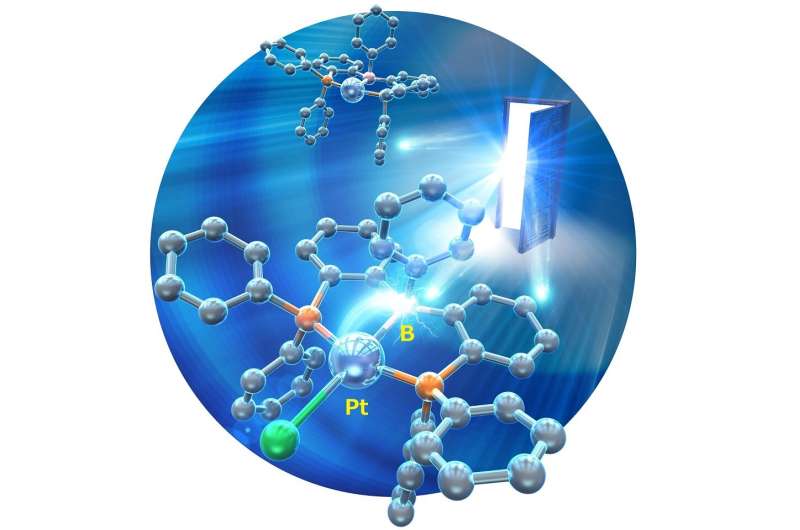
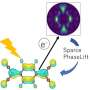
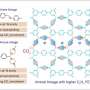
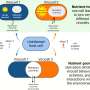
Extremely unstable anionic Pt0 complexes have been successfully stabilized by leveraging the electron-accepting ability of boron compounds. Credit: Hajime Kameo, OMU
Anionic M0 complexes (M = group 10 metals) have attracted attention as active species for catalytic reactions; however, their molecular structures have very rarely been determined due to their extremely high reactivity. Particularly, the structures of Pt0 complexes, which are expected to exhibit a high degree of reactivity, have not been determined, and their syntheses have been almost nonexistent.
Associate Professor Hajime Kameo, and Professor Hiroyuki Matsuzaka from the Osaka Metropolitan University Graduate School of Science and CNRS Senior Researcher Didier Bourissou (Paul Sabatier University—Toulouse III) elucidated the molecular structures of anionic Pt0 complexes for the first time. Their findings were published in Angewandte Chemie International Edition.
The key to success is the stabilization of anionic Pt0 complexes (which are usually unstable due to their electron-donating nature) by the electron-accepting properties of boron compounds.
"Although platinum complexes that exhibit a variety of catalytic activities have been actively studied, anionic Pt0 complexes have remained a mystery," stated Professor Kameo. "The results of this research not only enable us to elucidate the properties and functions of highly active chemical species but also provide new guidelines for their creation. It is expected to lead to the development of innovative catalytic reactions mediated by these chemical species."
More information: Hajime Kameo et al, Square‐Planar Anionic Pt0 Complexes, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202301509
Journal information: Angewandte Chemie International Edition
Provided by Osaka Metropolitan University