This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New metal-free porous framework materials may have potential for hydrogen storage
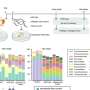
Researchers at the University of Liverpool and the University of Southampton have used computational design methods to develop non-metal organic porous framework materials, with potential applications in areas such as catalysis, water capture or hydrogen storage.
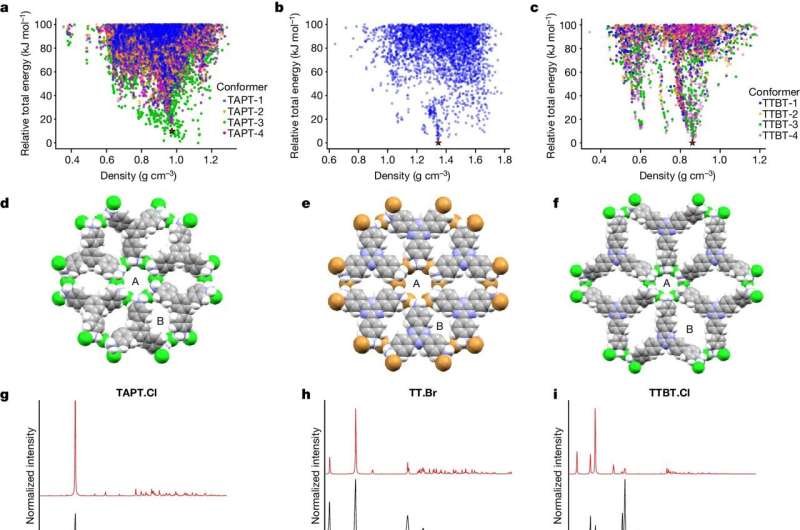
In a study published in the journal Nature, the research team used inexpensive and abundant non-metallic elements, such as chloride ions, to design non-metal organic porous frameworks (N-MOFs).
The new materials offer an alternative to metal-organic frameworks (MOFs), a class of porous, crystalline materials made up of metals connected by organic linker compounds.
More than 95,000 MOFs have so far been discovered with a broad range of applications in fields such as catalysis, gas separation and energy storage.
The new metal-free porous framework materials are yet to be fully explored but have already shown early promise for the capture of iodine, which is important in the nuclear industry. Other applications areas could include proton conduction, catalysis, water capture and hydrogen storage.
The research team think that in the future, it should be possible to extend the strategy to materials where organic linkers are connected by ions made up of other common non-metal elements such as nitrogen, oxygen and sulfur.
The research drew on complementary expertise in the discovery of new materials and robotics from the University of Liverpool alongside computational modeling expertise from the University of Southampton.
Professor Andrew Cooper from the University of Liverpool's Department of Chemistry and Materials Innovation Factory in Liverpool said, "This work opens up a range of possibilities. Our approach uses non-metal anions as nodes to build frameworks rather than metal cations in MOFs. There are more anions available than there are metals in the periodic table, so the space to search for new materials is huge."
However, there is a long-standing problem: metal nodes in MOFs direct the framework structure, rather like joints in a scaffold. These joints have a predictable geometry that allows MOFs to be designed for specific applications. This "molecular Lego" approach does not work for non-metallic salts because the interactions are much less directional.
Professor Graeme Day from the University of Southampton's School of Chemistry said, "We guided the discovery of these materials using a computational method called crystal structure prediction.
"This allows us to predict which non-metal salts will form stable porous frameworks, which salts will not, and to anticipate the precise crystal structure in advance of experimental work. We don't have to assume a specific geometry for the joints in the framework, which is a fundamental principle in MOF chemistry."
The research forms part of a broader program of research that aims to redefine the way that we discover new materials by combining emerging techniques in computational prediction, artificial intelligence, and robotics.
More information: Andrew Cooper, Porous isoreticular non-metal organic frameworks, Nature (2024). DOI: 10.1038/s41586-024-07353-9. www.nature.com/articles/s41586-024-07353-9
Journal information: Nature
Provided by University of Liverpool