Graphical abstract. Credit: Cell Reports Methods (2023). DOI: 10.1016/j.crmeth.2023.100419
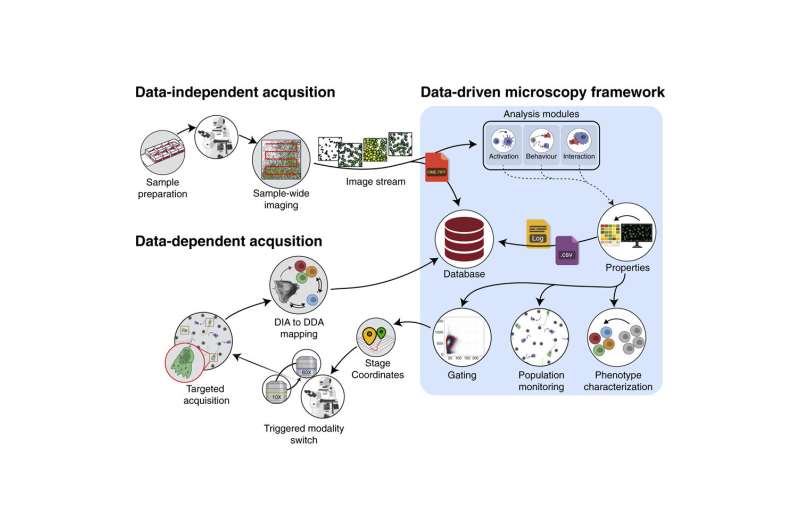
Is it possible to know exactly where to point a microscope in order to capture the precise moment a bacterium or a virus infects a cell? In order to take high resolution microscopic images of living biological material, you need to know exactly where to point the microscope. Researchers at Lund University in Sweden have now developed a software solution for smart, data-driven microscopy, which makes this possible. The research is published in Cell Reports Methods.
A challenge when producing images of living biological material such as cells is knowing how to align the microscope in order to capture the specific interaction you are interested in—such as the moment of infection, for example. It is difficult to know whether you are in the right place, and succeeding often requires many attempts, which can take months. Researchers at Lund University decided to find a solution, which they call smart microscopy.
"First, a low-resolution, rough scan of the specimen we wish to photograph is conducted, which provides data points about the specimen and a quick overview. Algorithms we have developed then calculate exactly where the motorized microscope should zoom in to capture what we are interested in—or something you may not have expected to see in the specimen being studied," says Pontus Nordenfelt, researcher in infection medicine at Lund University.
The method involves a software update of the ordinary microscope, and it has many applications. One area where researchers believe the solution will have an impact is for those who study cell migration, for example how cancer cells move around the body. A low-resolution image quickly captures datapoints on the cancer cells that are moving. This information is then used to calculate the coordinates of where the microscope is to film in high resolution. The result is a high-resolution film of the cells that are of greatest interest to study, for example in cancer research.
Another highly topical area in which the method can be used is in studies of the immune system. Here, it is possible to find either cells or microbes (e.g. bacteria, fungi or other microorganisms) that are behaving differently and automatically aim the microscope to film them in high resolution. Researchers are then able to analyze patient specimens and find abnormalities or find effective treatments. For example, an early variant of the method was used to find neutralizing antibodies against SARS-CoV-2.
Researchers suggest that as well as this saving time, the method also means that the right things are captured in a way that is possible to replicate. The method can be used to capture an image of the same individual cell in a specimen, but with different kinds of microscope.
"Normally, it is humans who decide which interaction should be captured and what is sufficiently good data. The fact is, however, that we are not all that good at doing that. With smart microscopy, you can derive from the data exactly what it is you want to collect, thereby removing the potential for human error from the collection process itself," says Oscar André, doctoral student at Lund University and first author of the article.
Researchers have tested their method on various scenarios and have now started a company, Cytely (.io) in order to further develop the concept and to give more people access to the technology.
More information: Oscar André et al, Data-driven microscopy allows for automated context-specific acquisition of high-fidelity image data, Cell Reports Methods (2023). DOI: 10.1016/j.crmeth.2023.100419
Journal information: Cell Reports Methods
Provided by Lund University