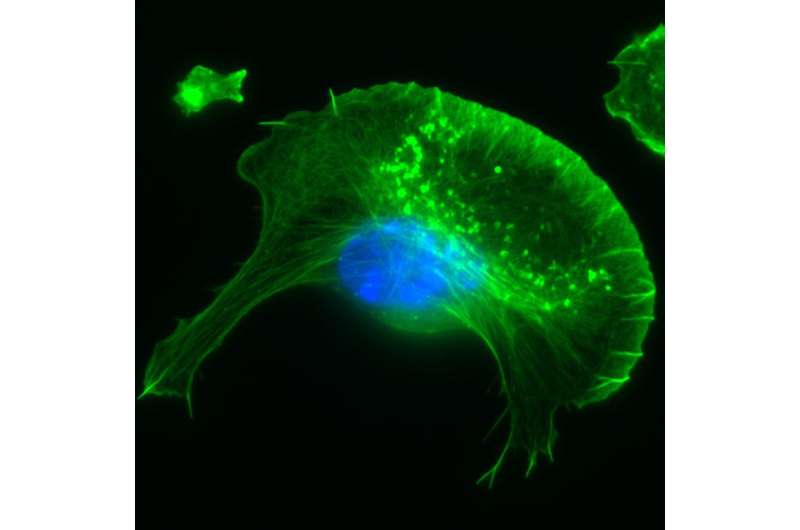
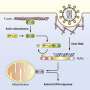
Actin cytoskeleton (green) of a migrating cell with nucleus (blue). Credit: HZI/Yubo Tang
Bacterial pathogens like Salmonella, Shigella, Listeria and many others exploit the protein skeleton of the cells they infect in order to spread throughout the host. However, how is this so-called cytoskeleton of host cells assembled and remodeled? Answers to this question can provide new approaches for combatting the causative agents of infectious diseases.
An international team of researchers including HZI-members have now unraveled precise molecular mechanisms of activation of a key signaling unit in cytoskeletal remodeling. These recent results have just appeared in Science Advances.
Cells in our body harbor a complex and frequently entangled network of filaments, which fulfills specific functions in maintaining or altering cell shapes during processes like embryonic development or migration of immune cells towards infectious agents. In addition, the cytoskeleton plays a central role in transport processes, both within cells and when crossing barriers between neighboring cells.
Therefore, it is not surprising that infectious agents have evolved numerous means to exploit the cytoskeleton of their hosts, which allows pathogens to enter host cells or to move across them to infect deeper tissue layers.
"A better understanding of the cytoskeleton and the underlying molecular mechanisms of its formation will allow more precise interventions with pathogenic processes," says Prof Klemens Rottner, head of the research group Molecular Cell Biology at the Helmholtz Center for Infection Research (HZI) in Braunschweig.
The international research project that has just appeared in the scientific journal Science Advances, was initiated by Prof Baoyu Chen from Iowa State University, U.S., and substantially supported by the HZI-team around Klemens Rottner.
The researchers were able to unearth novel, fundamental details concerning activation of the so-called WAVE Regulatory Complex (WRC). This protein complex assembles at the inner surface of the plasma membrane and operates like a central signaling unit. Upon activation, WRC triggers the formation of prominent cytoskeletal protrusions known as lamellipodia, which are key structures allowing the cell to migrate and engulf extracellular particles.
As opposed to a static skeleton, the subpart of the cytoskeleton comprising actin filaments is highly dynamic: It is constantly re-arranged and thus enables cells to develop locomotive forces to drive cell migration and change cell shape.
It was previously shown that WRC is responsible for the assembly of actin filaments in lamellipodia downstream of the signaling switch Rac1—a small GTPase. "That WRC activation was somehow connected to yet another GTPase, called Arf1, was also known," Rottner says. "In the current study, we wanted to understand how precisely Arf1 docks onto WRC and what happens in the context of WRC activation upon Arf1 binding."
Employing biochemical and structural biology approaches at Iowa State University and cell biological studies using CRISPR/Cas9 technology at HZI, together with diverse expertise from researchers at Stony Brook University, Mayo Clinic, and University of Pittsburgh, the team defined and characterized the Arf1 binding site on WRC and characterized its WRC-activating function.
"Specifically, WRC harbors three binding sites for signaling switches: two specific for Rac1 that were already established, and a novel one for Arf1. The latter is positioned between the two Rac-binding sites," Rottner says. The researchers could also show that Arf1 can only bind WRC after Rac1 binding to its so-called D-site on the complex.
"Rac1-binding to the D-site enables a conformational change on WRC, allowing interaction with Arf1. Such allosteric changes are famous phenomena in protein biochemistry, and in particular, in the context of WRC regulation," Rottner explains.
The researchers also established that optimal WRC activation occurs upon occupancy of all three GTPase binding sites. Together, the team was able to establish that aside from Rac1 binding, Arf1 constitutes a crucial signaling switch on WRC, required for its optimal function.
Such fundamental molecular insights as in this case on WRC regulation by Arf1 can be of potential interest for applied infection research. "Several previous studies have reported functions for Arf1 activation in host cell invasion by pathogenic bacteria—e.g., Salmonella. This was accompanied by increased actin remodeling enhancing by bacterial uptake," Rottner says.
"Arf1 or its binding site on WRC could thus constitute potential hubs for interfering with the pathogens' invasion strategies." Specific interference with this particular Arf1 mode of action might slow local actin remodeling and thus inhibit bacterial uptake. Rottner states, "We hope that our insights will inspire the development of novel ideas and strategies for the intervention with specific host-pathogen interactions."
More information: Sheng Yang et al, Arf GTPase activates the WAVE regulatory complex through a distinct binding site, Science Advances (2022). DOI: 10.1126/sciadv.add1412
Journal information: Science Advances
Provided by Helmholtz-Zentrum für Infektionsforschung