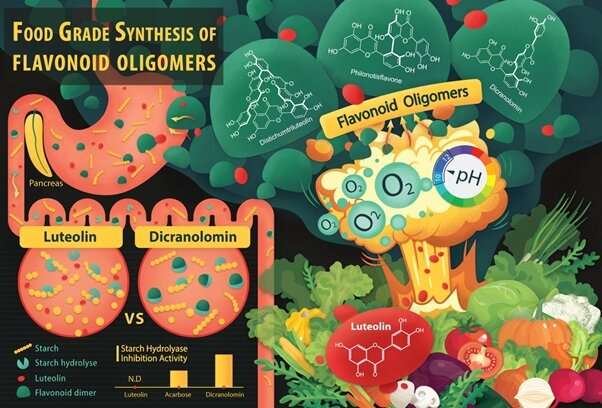
The food-grade synthesis of bi- and tri-flavones. Luteolin (flavone monomer) is a common flavonoid that exists in many types of plants including fruits and vegetables. By reacting with molecular oxygen in alkaline water, it forms flavone dimers and oligomers (Distichumtriluteolin, Philonotisflavone and Dicranolomin). Compared to Luteolin, the starting material, one of these more complex compounds, Dicranolomin shows strong starch hydrolase inhibition activity which can help to control an increase in the blood glucose level, especially after a meal. Credit: Yang Xin and Anna Zhou Yue
National University of Singapore food scientists developed a method to synthesize bioactive bi- and tri-flavones using molecular oxygen and food-grade alkaline water.
Flavonoids are bioactive compounds derived from plants that have a wide range of health promoting benefits. This includes reducing the risk of non-communicable diseases such as hypertension, diabetes and chronic inflammation, as well as battling infectious diseases such as urinary tract infection. While flavone monomers are relatively abundant in fruits and vegetables, the more potent flavonoid dimers and oligomers are often only present in trace amounts, making it impractical to extract them from natural sources. Due to their complex structures, it is also challenging to synthesize these molecules.
In a paper published in Nature Communications, Professor Huang Dejian from the Department of Food Science and Technology, National University of Singapore and his research team developed a catalyst-free method to drive the coupling reaction between flavone monomers to create flavone dimers and oligomers. They found that molecular oxygen acts as a hydrogen acceptor in alkaline water, allowing the coupling reaction to take place at room temperature and in the process, generate a high yield.
Using this method, the researchers synthesized more than 40 flavone dimers and trimers, some of which are completely new. This work is a research collaboration with Professor Kendall Houk from the University of California, Los Angeles.
Prof Huang said, "This reaction was developed from a serendipitous discovery by my doctoral student, Dr. Yang Xin. Paying attention to details, particularly unexpected results when conducting research, can lead to big discoveries."
This finding opens up an eco-friendly and efficient way to synthesize flavone dimers and oligomers with potential applications in battling disease and improving human health. These can potentially be used as active ingredients or pharmaceutical agents to fortify foods, produce nutritional supplements and treat diseases.
With the small library of bi- and tri-flavones synthesized from their newly discovered method, Prof Huang and his research team have been screening the bioactivity of these flavonoids including anti-viral, anti-aging, and starch hydrolase inhibition activities, as well as studying the relationship between the structure and activity of these flavonoids. They are also working to expand the scope of the food grade synthetic reaction to synthesize oligomers of other polyphenolic compounds.
"We welcome cooperation with interested partners in studying the bioactivities of the bi- and tri-flavones. Our team believes that the unique bi- and tri-flavones library will contribute to promoting human health and preventing disease," added Prof Huang.
More information: Xin Yang et al, Oxygen mediated oxidative couplings of flavones in alkaline water, Nature Communications (2022). DOI: 10.1038/s41467-022-34123-w
Journal information: Nature Communications
Provided by National University of Singapore