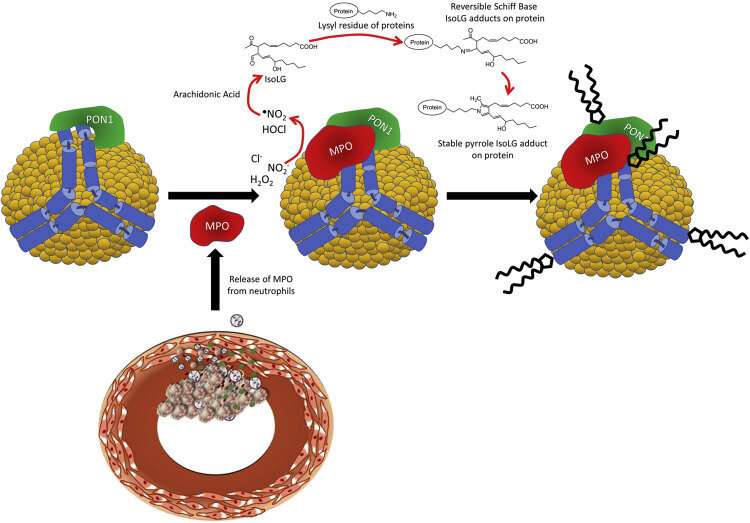
Figure 1. Schematic of PON1/MPO interaction with apoA-I on HDL and the production of IsoLG. Activated neutrophils at the site of atherosclerotic lesions release MPO, which associates with circulating HDL. MPO forms a ternary complex with apoA-I and PON1. MPO catalyzes the formation of reactive oxygen species such as hypochlorite which peroxidizes arachidonic acid to form IsoLG. IsoLG reacts extremely rapidly with primary amines such as the lysyl residues of HDL proteins like apoA-I to form covalent adducts. Credit: DOI: 10.1016/j.jbc.2021.101019
PON1 (paraoxonase 1), an enzyme associated with high-density lipoprotein (HDL), breaks down lipid peroxides, highly reactive fatty molecules that can damage blood vessels.
In this way, PON1 can protect against the development of cardiovascular disease. Numerous studies have observed reduced PON1 enzymatic activity in patients with cardiovascular disease.
Reporting last month in the Journal of Biological Chemistry, Sean Davies, Ph.D., and colleagues demonstrate in vitro that other reactive molecules called isolevuglandins (IsoLGs) can directly modify PON1, and that direct modification is the primary mechanism by which IsoLG reduces PON1 activity.
In collaboration with MacRae Linton, MD, and colleagues, the Davies group previously showed in a mouse model that scavenger molecules that bind to and remove IsoLGs from the bloodstream significantly enhanced PON1 activity.
While further studies in vivo are necessary to determine the extent to which IsoLG modification of PON1 contributes to reduced enzyme activity, the current study supports the notion that blocking this modification could prove beneficial to reduce atherosclerosis, the researchers concluded.
More information: Geetika Aggarwal et al, Myeloperoxidase-induced modification of HDL by isolevuglandins inhibits paraoxonase-1 activity, Journal of Biological Chemistry (2021). DOI: 10.1016/j.jbc.2021.101019
Journal information: Journal of Biological Chemistry
Provided by Vanderbilt University