Pd-catalyzed redox divergent coupling of ketones with terpenols. Credit: ZHAO Chaoyang
Hydrogenation and dehydrogenation are two important artificial methods to change the redox state of compounds. However, most current research focuses on mono redox transformation; the corresponding regulation of redox divergent couplings with artificial catalysts still remains challenging.
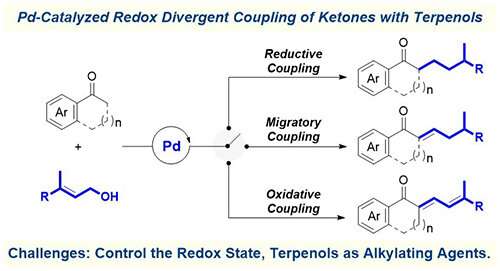
Recently, a team led by Prof. Chen Qing'an from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) developed a strategy to realize Pd-catalyzed redox divergent coupling of ketones with terpenols to access α-substituted ketones with varying degrees of unsaturation.
Their study was published in ACS Catalysis on May 27.
The researchers employed different additives to facilitate the control of oxidation states of the product, and found that reductive coupling pathway was thermodynamically favored with the aid of BnOH as an external hydrogen source.
They used LiBr as the additive to reduce the reactivity of Pd-H, in order to divert the selectivity toward α,β-unsatuated ketones. By switching the solvent from toluene to chlorobenzene, the active species Pd-H would be fully quenched to enable oxidative coupling.
"This redox divergent coupling protocol provides an important complement for known precedents on Tsuji-Trost allylation of ketones," said Prof. Chen.
More information: Chao-Yang Zhao et al, Pd-Catalyzed Redox Divergent Coupling of Ketones with Terpenols, ACS Catalysis (2021). DOI: 10.1021/acscatal.1c01488
Journal information: ACS Catalysis
Provided by Chinese Academy of Sciences