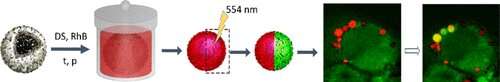
In modern biomedical science and developmental biology, there is significant interest in optical tagging to study individual cell behavior and migration in large cellular populations. However, there is currently no tagging system that can be used for labeling individual cells on demand in situ with subsequent discrimination in between and long-term tracking of individual cells. In this article, we demonstrate such a system based on photoconversion of the fluorescent dye rhodamine B co-confined with carbon nanodots in the volume of micron-sized polyelectrolyte capsules. We show that this new fluorescent convertible capsule coding system is robust and is actively uptaken by cell lines while demonstrating low toxicity. Using a variety of cellular lines, we demonstrate how this tagging system can be used for code-like marking and long-term tracking of multiple individual cells in large cellular populations.
Researchers from Skoltech and Saratov State University have designed a simple and easily reproducible labeling system for individual cells that enables researchers to track single cell behavior and migration for tasks requiring extreme precision. The paper was published in the journal ACS Applied Materials and Interfaces.
Modern biomedical science and developmental biology often require scientists to track and trace individual cells, whether it is to establish the best purified cells from various types of cell lines, in particular to select mesenchymal stem/stromal cells best suited for tissue regeneration or even to observe a parasite within a host. One way to do this is with a rainbow of various fluorescent proteins, shining in all kinds of colors under ultraviolet or blue light. However, this requires creating new transgenic lines capable of expressing these proteins and necessarily shifting away from the original cell cultures. Some of the proteins are also toxic and degrade quickly.
Instead of marker proteins, Gleb Sukhorukov of Skoltech and Queen Mary University of London and his colleagues decided to use polymer multilayer microcapsules, which many cells can impregnate and keep inside for days. The team built a hybrid polymeric microcapsule with carbon nanodots (CNDs) loaded with rhodamine B, a fluorescent dye; these components in combination makes the capsule photo-convertible.
The researchers tested their system on individual cell marking on six cell lines, including the famously immortal HeLa line and healthy human embryonic fibroblasts. The system worked quite well, with no tags decomposing even after 48 hours of observation and no traceable damage to the marked cells. And since a single cell can be marked with several clearly distinguishable microcapsules, this tool can be used for combinational coding of individual cells within populations.
"Although there were other approaches for single cell optical labeling, they were all based on genetic modification of the cells and encoding of photo-convertible proteins, which limits the application, especially on stem cells and other cells where genetic modification is not possible or not appropriate. Our approach gives a simple tool, applicable for various cell lines as photoconvertible capsules are readily internalized within cells. Another advantage is the possibilities for combinatorial labeling: in other words, each labeled cells could have a unique combination of switched capsules that gives a "code" to single cells and facilitates the monitoring of cell moves within population," Sukhorukov says.
He notes that the team expects biologists and biomedical researchers interested in solving problems of single cell movement and cell-to-cell communication in the populations to reach out and discuss possible solutions.
"Apart from that, we do not know much about how cells of the same type are communicating in the population, why some cell become 'leaders' as they are the first to respond to stimuli and others just follow. We believe that once we could select and demonstrate the biological or biochemical differences in individual cells in their population, our method should become more in demand and widespread," Sukhorukov concludes.
More information: Polina A. Demina et al, Fluorescent Convertible Capsule Coding Systems for Individual Cell Labeling and Tracking, ACS Applied Materials & Interfaces (2021). DOI: 10.1021/acsami.1c02767
Journal information: ACS Applied Materials and Interfaces
Provided by Skolkovo Institute of Science and Technology