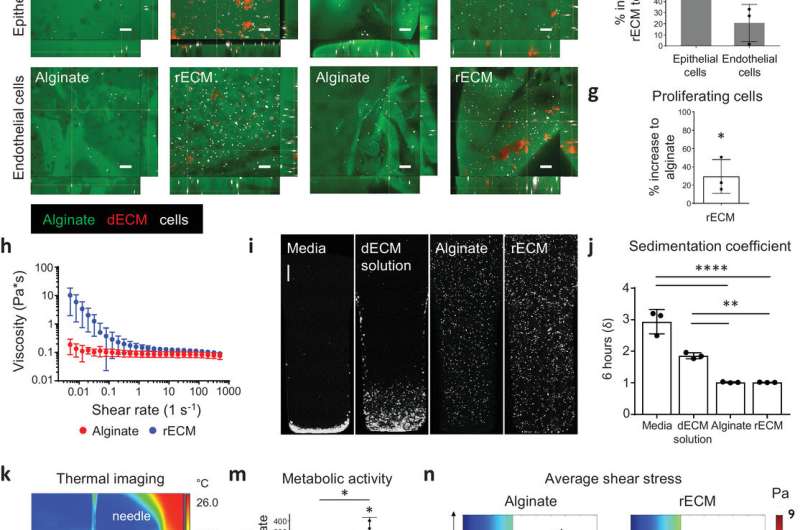
Characterization of rECM hybrid hydrogels. a) Picture of alginate and mouse rECM hydrogels. Scale bars: 1 mm. b) Alginate–fluorescein‐ and ECM–rhodamine‐modified rECM hydrogel showing the distribution of the alginate and ECM components within the hydrogel (see also Video S1 in the Supporting Information). Scale bar: 200 µm. c) SEM image of hydrogels. Scale bars: 50 µm. d) Strain crossover (%) between the storage and loss modulus in alginate hydrogels (2%) and rECM hydrogels (2% alginate, 5 mg mL−1 ECM) (n = 3 per group). e) Immunofluorescence images of murine lung epithelial MLE12 and endothelial bEnd3 (cells in white) in alginate–fluorescein (green) and ECM solution–rhodamine (red) modified rECM hydrogels on day 0 (day of seeding) and day 7. Scale bars: 100 µm f) Percent increase in metabolic activity of epithelial cells (MLE12) and endothelial (bEnd.3) cells in rECM hydrogels compared to alginate hydrogels on day 7 (n = 3 per group). g) Percent increase of EdU+ proliferating murine epithelial cells (MLE12) in rECM hydrogels compared to alginate hydrogels on day 5 (n = 3 per group). h) Oscillatory rheometry (n = 3 per group). i) Cell sedimentation confocal images and j) calculated sedimentation coefficient (δ) of A549 cells in DMEM–F12 cell culture media, alginate, mouse‐derived dECM and rECM solution for 6 h (n = 3 per group). Scale bar: 500 µm. k) Thermography of FRESH printing (see Video S2 in the Supporting Information). l) 3D bioprinted rECM hollow tube and branching structure (see Videos S3 and S4 in the Supporting Information). Scale bars: 2 mm. m) Metabolic activity (WST‐1 assay) on day 7 of seeded (in vitro) and 3D printed A549 cells in hydrogels (n = 3 per group). n) Average shear stress profiles of bioinks. Credit: Advanced Materials (2020). DOI: 10.1002/adma.202005476
Researchers at Lund University have designed a new bioink which allows small human-sized airways to be 3D-bioprinted with the help of patient cells for the first time. The 3D-printed constructs are biocompatible and support new blood vessel growth into the transplanted material. This is an important first step towards 3D-printing organs. The new study has been published in Advanced Materials.
Chronic lung diseases are the third leading cause of death worldwide with an EU cost of more than €380 billion annually. For many chronic diseases there is no cure and the only end-stage option for patients is lung transplantation. However, there are not enough donor lungs to meet clinical demand.
Therefore, researchers are looking at ways to increase the amount of lungs available for transplantation. One approach is fabricating lungs in the lab by combining cells with a bioengineered scaffold.
"We started small by fabricating small tubes, because this is a feature found in both airways and in the vasculature of the lung. By using our new bioink with stem cells isolated from patient airways, we were able to bioprint small airways which had multiple layers of cells and remained open over time," explains Darcy Wagner, associate professor and senior author of the study.
The researchers first designed a new bioink (a printable material with cells) for 3D-bioprinting human tissue. The bioink was made by combining two materials: a material derived from seaweed, alginate, and extracellular matrix derived from lung tissue.
This new bioink supports the bioprinted material over several stages of its development towards tissue. They then used the bioink to 3D-bioprint small human airways containing two types of cells found in human airways. However, this bioink can be adapted for any tissue or organ type.
"These next generation bioinks also support the maturation of the airway stem cells into multiple cell types found in adult human airways, which means that less cell types need to be printed, simplifying the nozzle numbers needed to print tissue made of multiple cell types," says Darcy Wagner.
Wagner notes that the resolution needs to be improved to 3D-bioprint more distal lung tissue and the air sacks, known as alveoli, that are vital for gas exchange.
"We hope that further technological improvements of available 3D printers and further bioink advances will allow for bioprinting at a higher resolution in order to engineer larger tissues which could be used for transplantation in the future. We still have a long way to go," she says.
The team used a mouse model closely resembling the immunosuppression used in patients undergoing organ transplantation. When transplanted, they found that 3D-printed constructs made from the new bioink were well-tolerated and supported new blood vessels.
"The development of this new bioink is a significant step forward, but it is important to further validate the functionality of the small airways over time and to explore the feasibility of this approach in large animal models," concludes Martina De Santis, the first author of the study.
More information: Martina M. De Santis et al. Extracellular‐Matrix‐Reinforced Bioinks for 3D Bioprinting Human Tissue, Advanced Materials (2020). DOI: 10.1002/adma.202005476
Journal information: Advanced Materials
Provided by Lund University