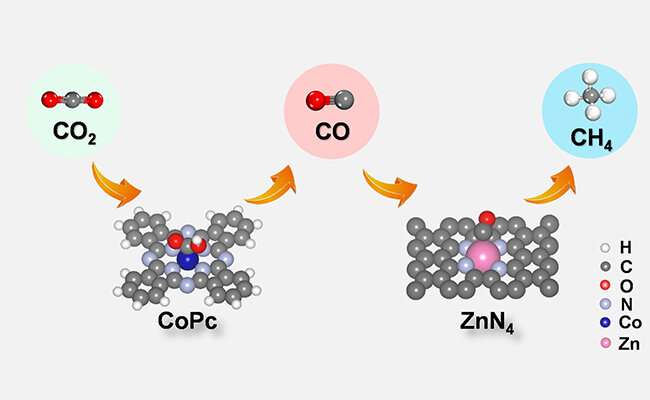
CO2 is first electrochemically reduced into CO and then CO diffuses to Zn-N-C for further conversion into CH4 over CoPc@Zn-N-C tandem catalyst. Credit: LIN Long and LIU Tianfu
Carbon dioxide reduction reaction (CO2RR) uses renewable electricity to convert carbon dioxide and water into fuels and chemicals, which is considered as an effective way to simultaneously realize carbon recycling and renewable energy storage.
The electrocatalytic conversion of carbon dioxide into hydrocarbons involves a multi-electron reduction reaction process, facing problems such as complex conversion pathways and difficulty in selectivity control.
A research group led by Prof. Wang Guoxiong and Prof. Bao Xinhe from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences enhanced CO2 electroreduction to methane with cobalt phthalocyanine (CoPc) and zinc-nitrogen-carbon (Zn-N-C) tandem catalyst.
They achieved high activity of CH4 production in CO2RR on a non-copper-based catalyst, and provided a new strategy for the electrocatalytic reduction of carbon dioxide to hydrocarbons.
This work was published in Angewandte Chemie International Edition on Sept. 4.
Compared with CoPc or Zn-N-C alone, the methane/carbon monoxide rate ratio of this CoPc and Zn-N-C tandem catalyst was increased by more than 100 times.
Density functional theory calculations and comparative experimental results showed that carbon dioxide was first reduced to carbon monoxide on the CoPc, and then the carbon monoxide diffused onto the Zn-N-C and was further converted into methane.
This tandem catalytic strategy converted carbon dioxide into methane and decomposed the process into tandem electrocatalytic reactions at two active sites. In this tandem catalytic system, CoPc provided carbon monoxide to retain the adsorbed hydrogen on the adjacent-nitrogen in the Zn-N site, thereby increasing the rate of methane production.
More information: Long Lin et al. Enhancing CO2 Electroreduction to Methane with Cobalt Phthalocyanine and Zinc‐Nitrogen‐Carbon Tandem Catalyst, Angewandte Chemie International Edition (2020). DOI: 10.1002/anie.202009191
Journal information: Angewandte Chemie International Edition
Provided by Chinese Academy of Sciences