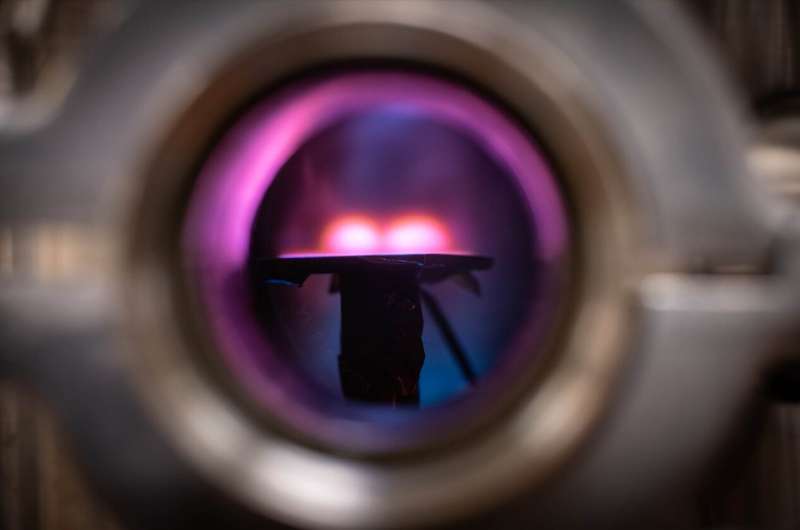
Hama Nadhom adjusts the gas supply to the vacuum chamber in which LiU researchers study how plasma electrons can be used to create thin metallic films. Credit: Magnus Johansson/Linköping University
Computers, mobile phones and all other electronic devices contain thousands of transistors linked together by thin films of metal. Scientists at Linköping University, Sweden, have developed a method that can use the electrons in a plasma to produce these films.
The processors used in today's computers and phones consist of billions of tiny transistors connected by thin metallic films. Scientists at Linköping University, LiU, have now shown that it is possible to create thin films of metals by allowing the free electrons in a plasma take an active role. A plasma forms when energy is supplied that tears away electrons from the atoms and molecules in a gas, to produce an ionized gas. In our everyday life, plasmas are used in fluorescent lamps and in plasma displays. The method developed by the LiU researchers using plasma electrons to produce metallic films is described in an article in the Journal of Vacuum Science & Technology.
"We can see several exciting areas of application, such as the manufacture of processors and similar components. With our method it is no longer necessary to move the substrate on which the transistors are created backwards and forwards between the vacuum chamber and a water bath, which happens around 15 times per processor," says Henrik Pedersen, professor of inorganic chemistry in the Department of Physics, Chemistry and Biology at Linköping University.
A common method of creating thin films is to introduce molecular vapours containing the atoms that are required for the film into a vacuum chamber. There they react with each other and the surface on which the thin film is to be formed. This well-established method is known as chemical vapour deposition (CVD). In order to produce films of pure metal by CVD, a volatile precursor molecule is required that contains the metal of interest. When the precursor molecules have become absorbed onto the surface, surface chemical reactions involving another molecule are required to create a metal film. These reactions require molecules that readily donate electrons to the metal ions in the precursor molecules, such that they are reduced to metal atoms, in what is known as a "reduction reaction." The LiU scientists instead turned their attention to plasmas.
"We reasoned that what the surface chemistry reactions needed was free electrons, and these are available in a plasma. We started to experiment with allowing the precursor molecules and the metal ions to land on a surface and then attract electrons from a plasma to the surface," says Henrik Pedersen.
A view into the vacuum chamber showing the plasma above the surface on which the metallic film is created. Credit: Magnus Johansson/Linköping University
Researchers in inorganic chemistry and in plasma physics at IFM have collaborated and demonstrated that it is possible to create thin metallic films on a surface using the free electrons in an argon plasma discharge for the reduction reactions. In order to attract the negatively charged electrons to the surface, they applied a positive potential across it.
The study describes work with non-noble metals such as iron, cobalt and nickel, which are difficult to reduce to metal. Traditional CVD has been compelled to use powerful molecular reducing agents in these cases. Such reducing agents are difficult to manufacture, manage and control, since their tendency to donate electrons to other molecules makes them very reactive and unstable. At the same time, the molecules must be sufficiently stable to be vaporised and introduced in gaseous form into the vacuum chamber in which the metallic films are being deposited.
"What may make the method using plasma electrons better is that it removes the need to develop and manage unstable reducing agents. The development of CVD of non-noble metals is hampered due to a lack of suitable molecular reducing agents that function sufficiently well," says Henrik Pedersen.
The scientists are now continuing with measurements that will help them understand and be able to demonstrate how the chemical reactions take place on the surface where the metallic film forms. They are also investigating the optimal properties of the plasma. They would also like to test different precursor molecules to find ways of making the metallic films purer.
More information: Hama Nadhom et al. Chemical vapor deposition of metallic films using plasma electrons as reducing agents, Journal of Vacuum Science & Technology A (2020). DOI: 10.1116/1.5142850
Provided by Linköping University