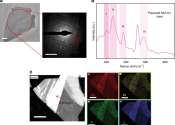
Towards novel promising perovskite-type ferroelectric materials: High-pressure synthesis of rubidium niobate
Capacitors are crucial components in electronic devices such as smartphones and computers. They are made of dielectric materials that polarize on the application of the voltage. Currently, barium titanate (BaTiO3) is the ...