This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Liquid-metal transfer from anode to cathode without short circuiting
University of Wollongong researchers achieved a significant milestone in novel soft-matter transport by demonstrating the transfer of liquid metal from an anode to a cathode without creating a short circuit, defying conventional expectations.
The team led by Prof Xiaolin Wang unveils a method where liquid-metal (specifically gallium-based, room temperature liquid metal) anodes can flow towards cathodes with a small electrical current without short circuiting.
The results, published in Nature Chemical Engineering last month, defies conventional electrochemical principles, and offers promising prospects for the development of shape-reconfigurable electrical conductors.
"The implications of this research extend to many potential applications," says Prof Wang. "Continuous back and forth transfer of liquid metal droplets, and the controllability of transfer, open new avenues for soft robotics and device engineering."
Avoiding short circuit
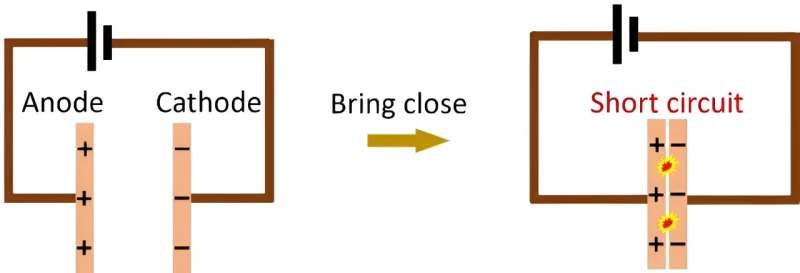
Conventionally, positively-charged anodes should short circuit when they are brought into contact with a cathode (see Figure 1).
The novel new approach allows liquid metal to flow from the anode towards the cathode without causing such electrical disruptions (see Figure 2).
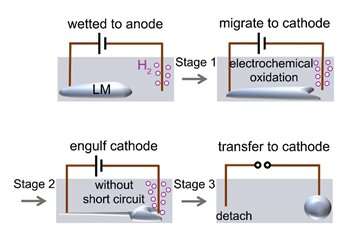
In the experiment, droplets of liquid metal attached to an anode move towards the cathode due to electrochemical oxidation, since electrochemical oxidation lowers the interfacial tension of the metal.
Typically, a solid electrode (for example, copper wire) is inserted into the liquid metal to apply the voltage that drives the electrochemical oxidation of the metal surface. The electrochemical reactions occur more intensely at the end of the metal closest to the cathode, creating a surface tension gradient (i.e., a Marangoni effect). The metal then migrates toward the opposite electrode.
"At this point, it would have been reasonable to expect a short circuit as the liquid metal completes the electrical circuit," says lead author Dr. Yahua He (UOW).
"However, in our experiment although the metal approaches and surrounds the counter electrode, it does not actually touch it, so there is no short circuit." The liquid metal continues to flow toward the cathode and surround it until finally the metal completely detaches from the anode and transfers to the cathode (see Figure 3a).
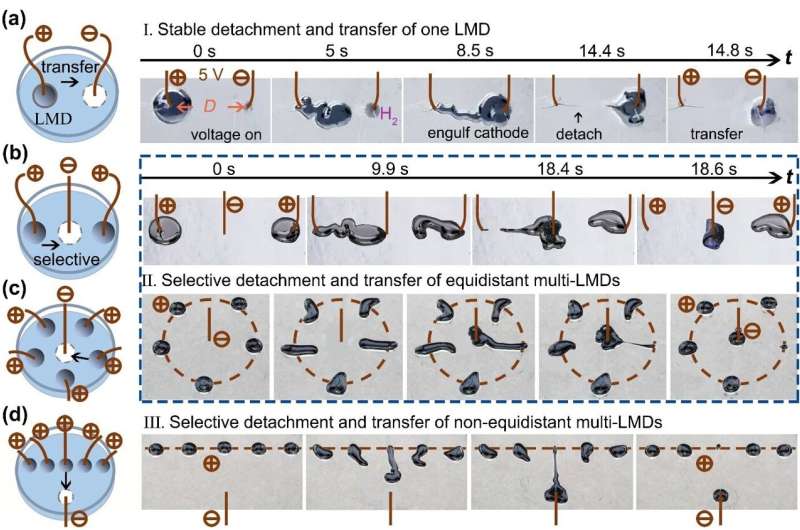
In summary, the short circuit is successfully avoided and enables the selective detachment and simultaneous transfer of liquid metal droplets from one electrode to another in aqueous media. A droplet can be selected to be completely detached from a metallic surface and simultaneously transferred to another metallic surface without short circuiting.
The bubble layer with critical thickness of 250 µm plays a dominating role to protect the liquid metal from short-circuiting and facilitate the smooth detachment and transfer process, while the oxides can also prevent the liquid metal from short-circuiting in dilute NaOH solution (≤0.25 M) with reduced liquidity.
Breaking it down drop by drop
The process is selective and depends on the distance between cathode and liquid metal; only the closest liquid metal droplet will detach and transfer (Figure 3b–e).
All liquid-metal-droplet anodes have the same potential and are thus all driven to move toward the cathode. Yet, for equidistantly arranged droplets (two droplets in Fig. 3b and five droplets in Fig. 3c), only one droplet can detach and transfer.
As shown in Figure 3b, two droplets are at equidistant sides of the cathode. They compete to deform and both move toward cathode. In this example, the left droplet arrives first at the cathode, then starts to surround the cathode, while the right droplet retracts to its initial position (a winner-take-all scenario). As a result, the left droplet fully detaches from the anode and is simultaneously transferred to the cathode. The right droplet stays at the initial position and remains attached to the copper wire.
For non-equidistantly arranged droplets in Figure 3d, only the droplet closest to the cathode selectively detaches and transfers. Thus, the transferred droplet can be selected by moving the cathode. This method only detaches and transfers one droplet at a time.
Moreover, after one droplet is transferred to the cathode, it can subsequently serve as a new cathode to detach and transfer another droplet. This capability enables a continuous transfer process for liquid metal systems with multiple drops.
Hydrogen and surface oxide provide screening
The underlying mechanisms behind this phenomenon involve hydrogen bubbles at the cathode, an ultra-thin surface oxide layer on the liquid metal, and a screening effect. These factors collectively prevent short circuiting and facilitate the selective detachment and transfer of liquid metal droplets.
When the metal approaches the cathode, three primary factors become important: 1) hydrogen bubbles at the cathode, 2) the surface oxide layer on the liquid metal, and 3) screening effect, as shown in Figure 4a–c.
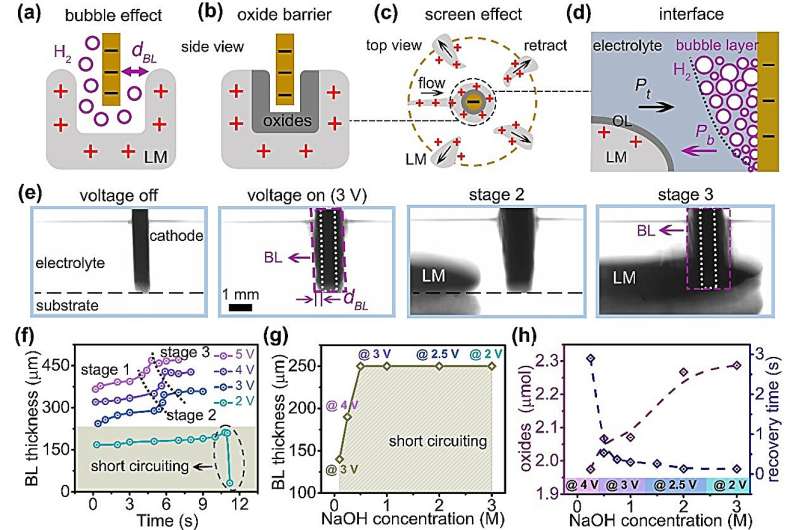
The first two factors physically block short circuiting (interface illustrated in Fig. 4d), while the third factor enables the selective detachment and transfer process of droplets. That is, when one liquid metal droplet surrounds the cathode, it screens the other droplets. As a result, other droplets terminate the oxidation process and retract to their initial positions.
Continuous transfer
Continuous back and forth transfer of liquid metal droplets can be realized by reversing the polarity of the electrodes.
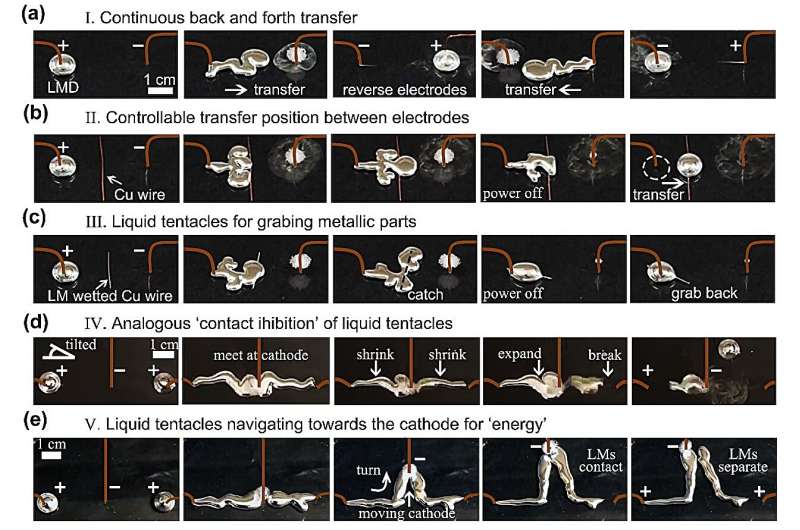
As shown in Figure 5a, when the electrodes are reversed after liquid metal has been completely transferred to the cathode, the liquid metal moves back to the initial position. Moreover, the transfer position is controllable by placing a copper wire between electrodes, as shown in Figure 5b.
When the copper wire is wetted by LMD, it merges with the wire in a shorter time compared to non-wetted metallic wire. Then, the LMD can easily grab the wire and pull it back toward the original position, like liquid tentacles (Figure 5c).
When two LM tentacles are arranged to arrive at the cathode at the same time by placing the cathode closer to the right droplet while slightly elevating the left side of petri dish, an analogous "contact inhibition" is observed in Figure 5d.
When the LMDs meet with each other at the cathode, they keep flowing from both anodes to the single cathode. When one LMD breaks from its anode, the other then quickly expands through oxidation. Moreover, the LM tentacles will navigate towards the moving cathode for "energy," analogous to the biological phenomenon chemotaxis. The cathode attracts the LMDs due to the gradients of the interfacial tension.
The LM tentacles could even turn to follow the cathode toward the power source, as shown in Figure 5e. The LM tentacles are able to contact or separate to each other by moving the cathode.
Applications
Such manipulation may expand useful strategies for liquid metals as shape-reconfigurable conductors for devices and actuators for soft robotics.
Moreover, the avoidance of short circuiting has implications for electrochemical engineering, such as the pronounced impact on the convective transport of electrochemically active species as well as on heat transfer near electrodes.
This research not only defies conventional electrochemical principles but also offers promising prospects for the development of shape-reconfigurable conductors and actuators. The avoidance of short-circuiting bears significant implications for electrochemical engineering, highlighting the profound impact on convective transport of electrochemically active species and heat transfer near electrodes.
More information: Yahua He et al, Liquid-metal transfer from an anode to a cathode without short circuiting, Nature Chemical Engineering (2024). DOI: 10.1038/s44286-024-00045-1
Journal information: Nature Chemical Engineering
Provided by FLEET