This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists find new paths to steer and optimize electrochemical processes
Optimizing electrochemical reactions is essential for the transition to renewable energies. In electrochemical reactions, electric currents and potential differences are used to binding and induce reactions. Electrochemistry is a pre-requisite for hydrogen production, and for battery technology, and thus for sustainable chemistry.
Although there has been a lot of technological development in this area in recent years, there is still room for improvement and a long way towards large scale industrial applications.
Scientists from the Cluster of Excellence RESOLV at the Ruhr University Bochum and École normale supérieure in Paris discovered two new aspects to control and thus optimize electrochemical reactions at electrified interfaces.
They describe their results in the Journal of the American Chemical Society.The article has been chosen by the journal to be featured on the front cover.
Surface sensitive spectroscopy
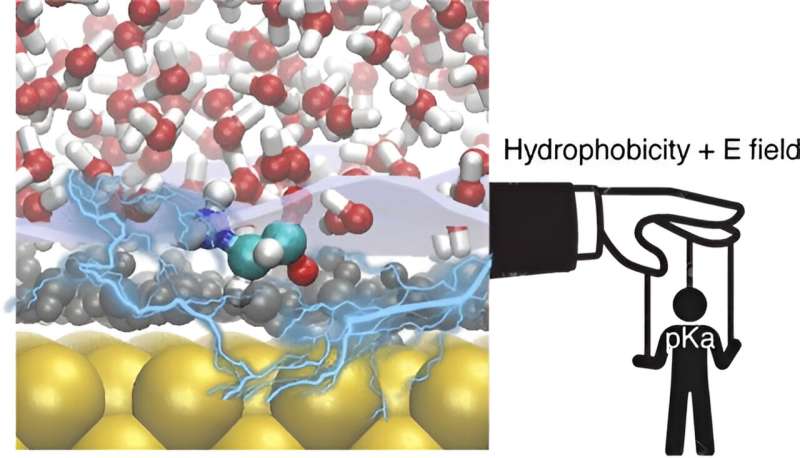
In order to understand the complex behavior at electrified interfaces, the team examined a critical parameter, called the acid dissociation constant (pKa) of molecules at electrified metal/water interfaces. Whereas in bulk solutions, this value is well known, it has been speculated that this parameter, which is essential for acid/base chemistry can be quite different in the vicinity of electrodes. However, measuring pKa values under electrochemical conditions is experimentally challenging.

To address this, the group of Havenith have combined advanced surface specific spectroscopic techniques, notably Surface-Enhanced Raman Spectroscopy (SERS), with theoretical modeling. The results vary with the applied voltage: Acid-base chemistry at electrified interfaces, is clearly different from chemistry in the bulk solution.
Hydrophobic layer and strong electric fields
Their findings highlight two key mechanisms governing acid-base reactions at electrified interfaces: The influence of local hydrophobicity and the impact of strong local electric fields. By analyzing the protonation/deprotonation of glycine molecules, the researchers observed a hydrophobic water/water interface close to the metal surface, leading to a destabilization of zwitterionic forms of glycine. When increasing the applied potential the effect is amplified.
Their results showcase the changes of local solvation properties at metal/water interfaces, presenting new avenues for fine-tuning reactivity in electrochemistry. These insights offer new opportunities for optimizing electrochemical processes and designing novel strategies for catalysis as both factors can be tuned in a controlled way.
More information: Steffen Murke et al, Tuning Acid–Base Chemistry at an Electrified Gold/Water Interface, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.3c13633
Journal information: Journal of the American Chemical Society
Provided by Ruhr-Universitaet-Bochum



















