This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Light-activated system enables controlled antigen release for analysis of antigen processing
The immune system is always on alert, detecting and eliminating pathogens and cancer cells. Cellular control mechanisms cause diseased cells to present antigens on their surface like signs for the immune system.
For analysis of the necessary complex antigen processing and transport processes in real time, a German team has developed a "cage" that is opened with light to release trapped antigens at a specific place and time. The study is published in the journal Angewandte Chemie International Edition.
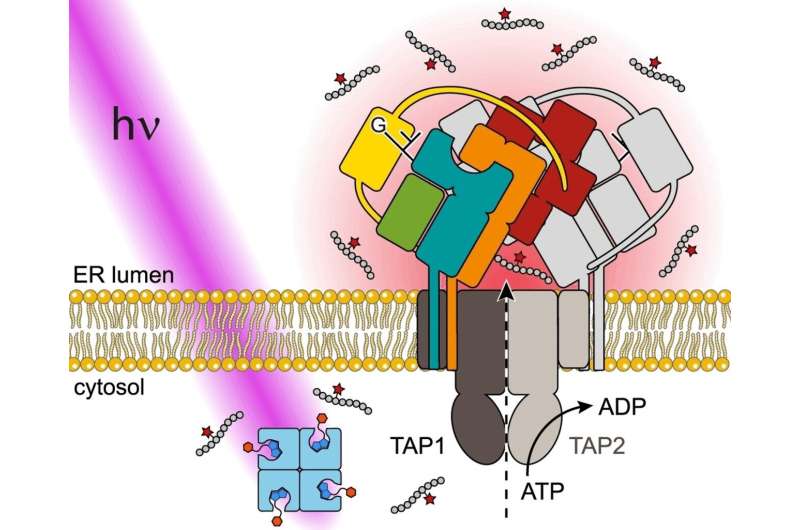
In our cells, both endogenous and foreign proteins are constantly broken into tiny pieces and transported into the endoplasmic reticulum (ER), a branched system of channels enclosed by a membrane, by the transporter associated with antigen processing (TAP). There, the supramolecular peptide loading complex PLC controls the loading of MHC I (major histocompatibility complex class I) with antigenic peptides.
Certain peptides are preferentially loaded onto MHC I, further processed for immune surveillance (antigen processing) and presented on the cell surface. Peptides that come from normal endogenous proteins remain immunologically inconspicuous (barring misdirected autoimmune reactions).
Despite many new insights, the mechanistic principles of antigen translocation, dynamic PLC assembly, and the interaction between different PLC subunits in the "quality control" of peptide-MHC complexes remain generally unknown.
To further analyze antigen processing, Ralph Wieneke, Robert Tampé, and their team at the University of Frankfurt am Main (Germany) have now developed a photostimulated antigen release system that can be used to precisely study antigen flux.
The antigens are released (antigen burst) on command from a "caged" inactive state by the application of light. The advantage of light stimulation is that it can be precisely dosed at limited times and locations and is noninvasive, which allows for experiments in living cells.
The team used a peptide derived from an HIV antigen as their model. They used a linker to bind the epitope (section of an antigen) to biotin and then the biotin to a voluminous protein called streptavidin. In this state, the epitope is shielded to the extent that it can no longer be recognized by the antigen processing transporter (TAP).
The linker contains a group that can be split apart by light. When irradiated with UV light, the peptide epitope is immediately released from its "cage." It is then recognized by TAP and transported across the ER membrane and loaded onto MHC I by way of the PLC.
This method is versatile, as demonstrated by a variety of scenarios, such as tracing antigen transport by the TAP of native human PLC in the ER membrane of a human lymphoma cell line.
According to Wieneke and Tampé, "It is our aim to use the light-activated system to follow the antigen processing pathway through different cellular compartments and gain an understanding of the kinetics of various immunological processes in vivo."
More information: Max Löffler et al, Antigen Delivery Controlled by an On‐Demand Photorelease, Angewandte Chemie International Edition (2024). DOI: 10.1002/anie.202405035
Journal information: Angewandte Chemie International Edition
Provided by Wiley