This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
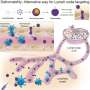
An efficient protein delivery system with spider minor ampullate silk protein nanoparticles
In a study published in the journal MedComm, researchers have developed an efficient protein delivery carrier based on spider silk proteins (spidroins), derived from Araneus ventricosus minor ampullate silk protein (MiSp). The MiSp-based nanoparticles are able to serve as an effective delivery system with high loading efficiency and a sustained release profile at physiological pH.
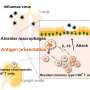
Under subcutaneous or intramuscular administration, protein antigen carried by these nanoparticles could enhance antigen-specific immune responses in mouse models, through a mechanism involving antigen-depot and long-term antigen persistence effect at the injection site, as well as efficient internalization by lymph nodes and uptake by dendritic cells.
Protein vaccines are often poorly immunogenic due to rapid degradation by proteases or promoting tolerance. As a potential alternative, nanoparticles as carriers can deliver antigens to antigen-presenting cells (APCs) and regulate the antigen presentation pathway or act as immune potentiators, enhancing subsequent antigen-specific immune responses.
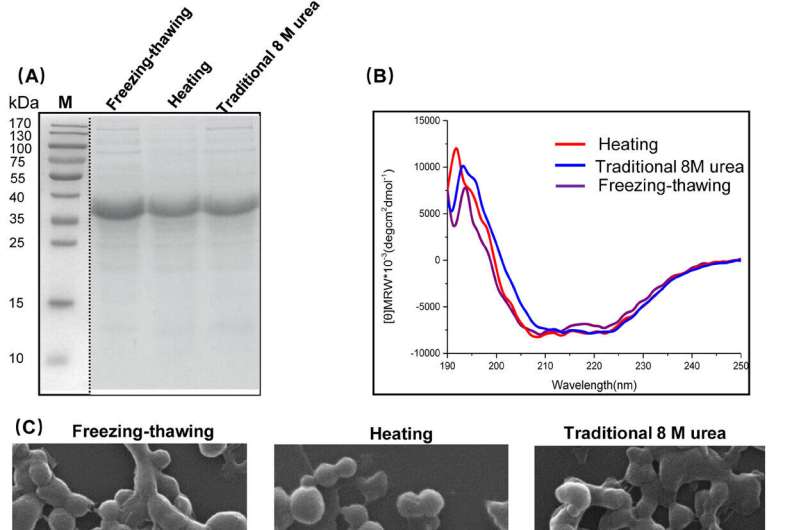
Among the particle-based carriers, spidroins have attracted significant attention due to their desirable characteristics, including biocompatibility, biodegradability, and the ability to induce only a mild immune response. In particular, they could be easily prepared by salting out with a potassium phosphate solution and sterilized with steam. Also, their size, stability, and drug loading/release kinetics are able to be easily tailored by adjusting the particle formation process and post-treatment of the material.
There are at least seven different types of spidroins that form a variety of silk types, each with unique properties suitable for diverse biological applications. This study focuses on the spider minor ampullate silk, since it is mechanically strong, does not super-contract in water, and fibrillizes through a non-nucleation dependent pathway.
The team designed a customized spidroin covering a globular N-terminal domain and a repetitive region for generation of nanoparticles. They first investigated the influence of protein inclusion body solubilization methods on sphere properties, and then selected lysozyme as a model antigen to study the delivery efficiency under different administration routes, including parenteral [subcutaneous (s.c.), intramuscular (i.m.), and intravenous (i.v.) injections] and non-parenteral (per-oral) administration routes.
This study demonstrates that the MiSp-based nanoparticle is a promising candidate for delivering antigen safely and effectively under various administration routes. It highlights how spider silk nanoparticles could be a powerful tool in medicine, particularly for delivering vaccines effectively and safely.
This study was led by Dr. Xingmei Qi and Dr. Sidong Xiong (Soochow University, China) and Dr. Gefei Chen (Karolinska Institutet, Sweden).
More information: Hairui Yu et al, Spider minor ampullate silk protein nanoparticles: an effective protein delivery system capable of enhancing systemic immune responses, MedComm (2024). DOI: 10.1002/mco2.573
Provided by Sichuan International Medical Exchange and Promotion Association