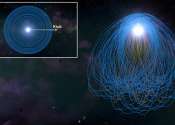
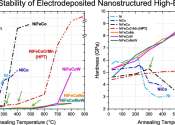
Hungry, hungry white dwarfs: Solving the puzzle of stellar metal pollution
Dead stars known as white dwarfs, have a mass like the sun while being similar in size to Earth. They are common in our galaxy, as 97% of stars will eventually become white dwarfs. As stars reach the end of their lives, their ...