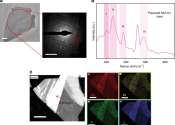
Scientists develop novel liquid metal alloy system to synthesize diamond under moderate conditions
Did you know that 99% of synthetic diamonds are currently produced using high-pressure and high-temperature (HPHT) methods? A prevailing paradigm is that diamonds can only be grown using liquid metal catalysts in the "gigapascal ...