Drexel researchers discovered that packing more polymer "bristles" onto a molecular bottle brush polymer causes it to bend, which is the foundation of spherical crystal growth. Credit: Drexel University
From snowflakes to quartz, nature's crystalline structures form with a reliable, systemic symmetry. Researchers at Drexel University, who study the formation of crystalline materials, have shown that it's now possible to control how crystals grow—including interrupting the symmetrical growth of flat crystals and inducing them to form hollow crystal spheres. The discovery is part of a broader design effort focused on the encapsulation of medicine for targeted drug treatments.
The new development, recently reported in the scientific journal Nature Communications, was led by Christopher Li, Ph.D., a professor in Drexel's College of Engineering whose research in the Department of Materials Science and Engineering has centered around engineering polymer structures for special applications, in collaboration with Bin Zhao, Ph.D., a professor in the Chemistry Department at the University of Tennessee, Knoxville. Their work shows how these structures, like polymer crystal spheres, can be formed simply by mixing chemicals in a solution—rather than physically manipulating their growth.
"Most crystals grow in a regular pattern, if you think about snowflakes, there is a translational symmetry that guides the unit cell repeating throughout the crystalline flake. What we've discovered is a way to chemically manipulate the macromolecular structure so that this translational symmetry is broken when the molecule crystallizes," Li said. "This means we can control the overall shape of the crystal as it forms—which is a very exciting development, both for its scientific significance and the implications it could have for mass production of targeted therapies."
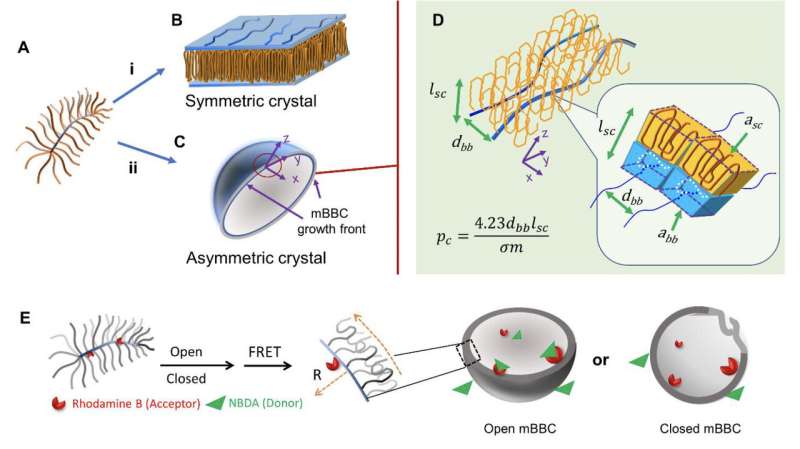
The technique Li uses to compel what would normally be a flake-like crystal to draw itself up into a sphere builds on his previous work with polymers that look like brushes and polymer crystals formed from emulsion droplets. Incorporating these pliable "bottle-brush" polymers as the structural system of the crystal, allows Li to shape its growth by adjusting the "bristles" of the brush.
By adding more polymer "bristles" to bottle brush polymers (left), researchers have discovered a way to initiate and pause the self-assembly of crystals from solution. The crystals could one day be used to encapsulate medicine for targeted drug therapies. Credit: Drexel University
"A bottle-brush polymer has lining bristles surrounding a spine, what we discovered is that we can make that spine bend upon crystallization by packing bristles on to one side of it," Li said. "This sets the pattern that is repeated as the crystal grows—so instead of growing flat it curves three-dimensionally to form a sphere."
This means the amount of bristle polymers in the solution will determine how much the bottle-brush spine bends and thus the shape and size of the crystal ball.
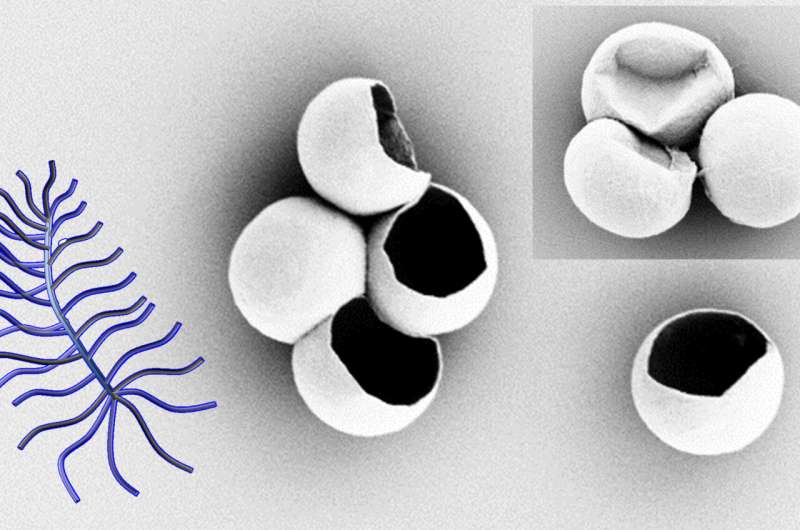
Li's team also reports on how to pause the formation of the crystal, leaving holes in the sphere that could be useful for inserting a medicinal payload during the manufacturing process. Once filled, it can be closed with polymers tailored to help direct it to its target in the body.
"We have been working toward this achievement for some time," Li said. "This spherical crystallography manifest itself in robust structures that we see in nature from egg shells to virus capsids, so we believe it is the ideal form to survive the rigors of delivering medication in the body. Being able to control the properties of the crystal as it forms is an important step toward realizing this application."
More information: Hao Qi et al. Breaking translational symmetry via polymer chain overcrowding in molecular bottlebrush crystallization, Nature Communications (2020). DOI: 10.1038/s41467-020-15477-5
Journal information: Nature Communications
Provided by Drexel University