The future of medicine could be found in this tiny crystal ball
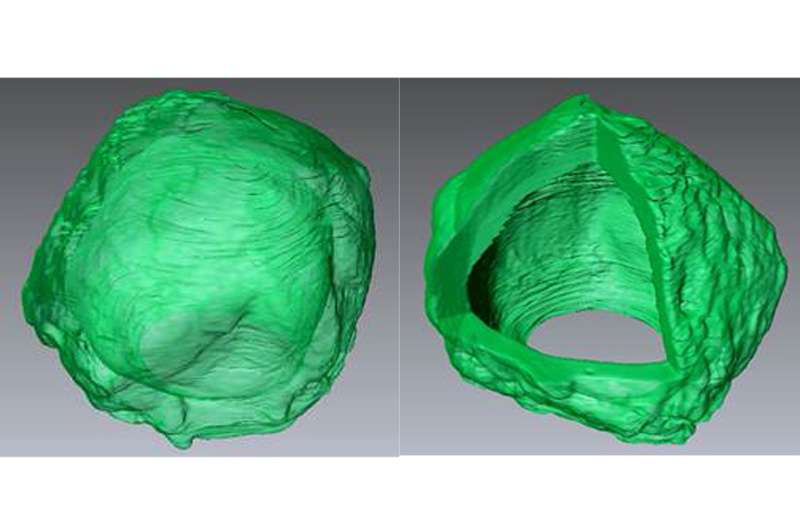
A Drexel University materials scientist has discovered a way to grow a crystal ball in a lab. Not the kind that soothsayers use to predict the future, but a microscopic version that could be used to encapsulate medication in a way that would allow it to deliver its curative payload more effectively inside the body.
Christopher Li, PhD, a professor in the College of Engineering and head of the Soft Materials Lab in the Department of Materials Science & Engineering, recently reported his finding in Nature Communications. It's a significant one, because up until now, crystals have grown in rigid, structured formations. One of the forms we're most familiar with is the snowflake, with a web of straight lines connecting to making a grid that grows into the crystalline flake.
Crystals form this way because their molecules are predisposed to align themselves in a way that links them via the strongest electrochemical bond available. If molecules are floating freely, as they are in a water vapor for example, they are able to follow this default course to connect with other molecules and, eventually, form a crystal—an ice crystal, or snowflake, in the case of water molecules.
But, as the adage holds: no two snowflakes are alike. This is because the formation of a crystal can be affected by the environment in which it forms. Li uses this molecular property to engineer his hollow crystal spheres.
His "crystalsomes" are named for their similarity to liposomes—tiny bubbles with the same membrane as cells that are being explored for use as biological packages for delivering drug treatments. But Li and his team estimate that their crystalsomes could actually be better at making the delivery than their namesake, because crystals hold up a bit better than liposomes both on the way into and within the body.
"Mechanical properties of polymeric materials could be improved by forming crystalline structures," Li said. "While precisely tuning crystallization within a nanoscale curved space is challenging, we envisage that this novel structure could shed light on investigating spherical crystallography and drug delivery."
Li was able to overcome crystal's edge-forming tendencies by containing it inside a droplet. A rough equivalent of this would be forcing a single snowflake to form inside a tiny snow globe, rather than in the open expanse of the atmosphere.
For his nanoscopic version of the snow globe, Li employed a little trick that you might recognize if you've ever tried to make vinaigrette. He created a tiny bubble of oil to encase water molecules. When the surfactant bubble was cooled to the appropriate temperature, the molecules inside began to crystalize. But rather than forming an angular web of connections, the molecules, instead, lined up along the interior of the oil bubble—crystallizing in a hollow, spherical shape.
Early tests indicate that the crystalsome is a few hundred times stronger than liposomes, which makes them a sturdier option for medicine encapsulation. With funding from the National Science Foundation, Li's team is now exploring ways to control the shape and strength of the spheres by making them out of different molecules.
More information: Wenda Wang et al. Highly robust crystalsome via directed polymer crystallization at curved liquid/liquid interface, Nature Communications (2016). DOI: 10.1038/ncomms10599
Journal information: Nature Communications
Provided by Drexel University