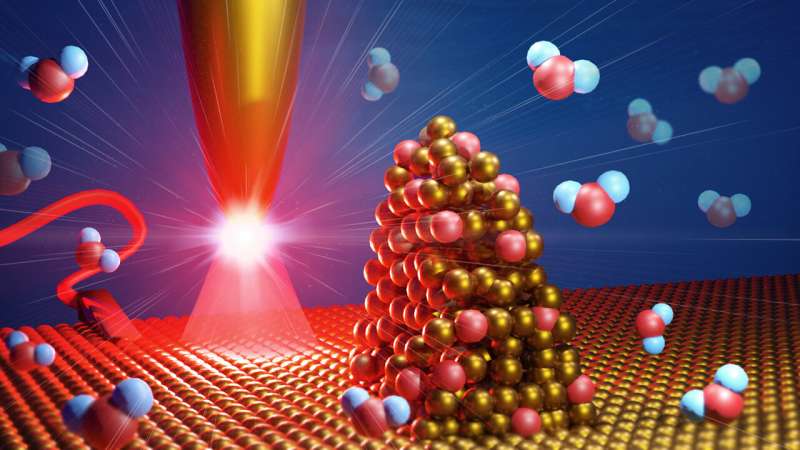
At rough areas of a catalyst surface, water is split into hydrogen and oxygen in a more energy efficient way than at smooth areas. Credit: MPI-P, License CC-BY-SA
It is a well-known school experiment: Applying a voltage between two electrodes inserted in water produces molecular hydrogen and oxygen. Researchers seek to make water splitting as energy-efficient as possible to advance industrial applications. The material of the electrode and its surface quality are crucial aspects that determine splitting efficiency. In particular, rough spots of only few nanometers in size, called reactive centers, determine the electrochemical reactivity of an electrode.
Previous investigation methods were not accurate enough to follow chemical reactions taking place at such reactive centers on the electrode surface with sufficient spatial resolution under real operating conditions, i.e., in electrolyte solution at room temperature and with an applied voltage. A team of scientists led by Dr. Katrin Domke at the MPI-P has now developed a method with which the initial steps of electrocatalytic water splitting on a gold surface could be studied for the first time with a spatial resolution of less than 10 nm under operating conditions.
"We were able to show experimentally that surfaces with protrusions in the nanometer range split water in a more energy-efficient way than flat surfaces," says Katrin Domke. "With our images, we can follow the catalytic activity of the reactive centers during the initial steps of water splitting."
The researchers combined different techniques: In Raman spectroscopy, molecules are illuminated with light that they scatter. The scattered light spectrum contains information that provides a chemical fingerprint of the molecule, enabling the identification of chemical species. However, Raman spectroscopy typically produces only very weak and spatially averaged signals over hundreds or thousands of nanometers.
For this reason, the researchers combined the Raman technique with scanning tunneling microscopy. By scanning a nanometer-thin gold tip illuminated with laser light over the surface under investigation, the Raman signal is amplified by many orders of magnitude directly at the tip apex, which acts like an antenna. This strong enhancement effect enables the investigation of isolated molecules. Furthermore, the tight focusing of the light by the tip leads to a spatial optical resolution of less than ten nanometers. Notably, the apparatus can be operated under realistic electrocatalytic operating conditions.
"We were able to show that during water splitting at nanometer rough spots—i.e., reactive centers—two different gold oxides are formed that could represent important intermediates in the separation of the oxygen atom from the hydrogen atoms," says Domke. The researchers have gained more precise insights into the processes taking place on the nanometer scale on reactive surfaces, which could facilitate the design of more efficient electrocatalysts in the future that require less energy to split water into hydrogen and oxygen.
The scientists have published their results in the journal Nature Communications.
More information: Jonas H. K. Pfisterer et al, Reactivity mapping of nanoscale defect chemistry under electrochemical reaction conditions, Nature Communications (2019). DOI: 10.1038/s41467-019-13692-3
Journal information: Nature Communications
Provided by Max Planck Society