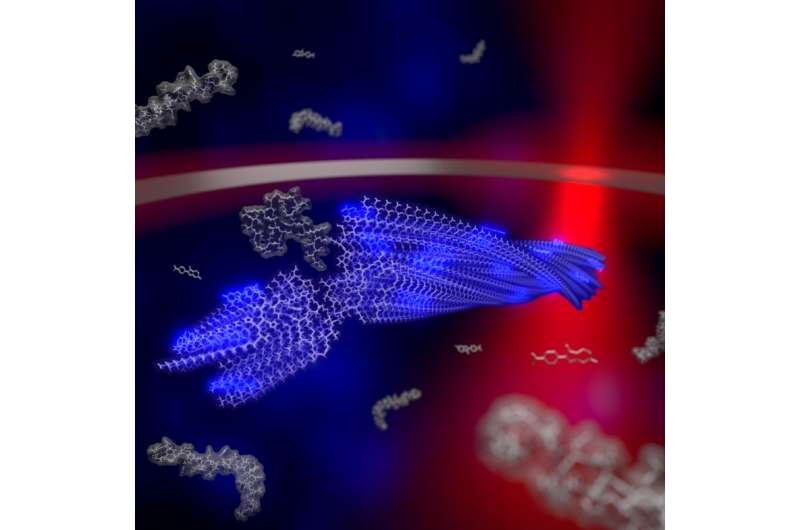
Illustration of a growing fibril in the trap and the laser. Credit: Martin Fränzl
More than 24 million people worldwide suffer from neurodegenerative diseases such as Alzheimer's, Parkinson's or Huntington's. The molecular causes of these diseases have so far been little investigated. A team of scientists from Leipzig University and the Technical University of Dresden, as well as the Kurt Schwabe Institute Meinsberg, is now looking into these molecular mechanisms with new approaches, and has developed a technique involving a thermal molecule trap. The researchers have published their findings in Nature Methods.
Researchers assume that the cause of these neurodegenerative diseases is the aggregation of small protein molecules called peptides. Peptides usually perform different tasks in the body with their special three-dimensional structure. For example, they act as hormones, they are involved in the transport of substances through the cell membrane, and have antibiotic and antiviral functions. However, when peptides come together to form small aggregates or even larger insoluble structures called plaques or amyloids, their original function is lost, and peptide aggregates can be toxic.
The way in which individual peptides become smaller aggregates and finally fibrils is not clear and experimentally difficult to observe. Even the growth of fibrils has not been sufficiently resolved since almost all previous studies have only been carried out for large quantities of molecules consisting of a mixture of peptides, aggregates and fibrils of different sizes.
The researchers have come up with new explanatory approaches: "When examining mixtures of single molecules, aggregates and fibrils for their properties, one obtains a picture of many overlapping effects. An important step towards a detailed understanding at the molecular level is to study the growth of individual amyloid fibrils," explains Prof. Dr. Frank Cichos, head of the project at Leipzig University.
Using their newly developed thermal trap, the researchers trapped individual fibrils in physiological solutions for several hours under the microscope and for the first time, observed the growth of the fibril, its breakup and the further growth of the fragments. "Developing a technique for this purpose was a tricky task. Molecules in liquids move steadily due to the temperature of the liquid. This so-called Brownian motion quickly drives them out of our field of observation, and we can only observe individual fibrils for a very short time," says Martin Fränzl, a doctoral candidate in the project.
The researchers are now harnessing the thermal energy that causes Brownian motion to trap the fibrils in a small volume. "We use a laser to heat up a tiny metal ring inside which the aggregates are trapped. The resulting temperature differences in the solution with the peptides drive them in any direction that we specify," explains Tobias Thalheim, who worked with Martin Fränzl on the thermal traps. But the trapping of the amyloids is not enough. The temperature-controlled trap also allows the scientists to track and mathematically analyze the movement of the fibrils. With the help of the rotational movement of the fibrils, they observed the change in size of the fibrils down to a millionth of a centimeter, and thus precisely determined their growth rate.
"We can now see processes that were previously assumed, but for which there was no direct experimental evidence," explains Cichos. For the growth of the fibrils, their breakage should play an important role, since it doubles the number of free ends where growth continues. The experiments show that fibrils break and thus form new sprouts, which help the peptides aggregate faster. "There is now a multitude of new experiments that are possible, and we can follow paths that were previously not possible," says Cichos.
Prof. Dr. Michael Mertig from the Technical University of Dresden, director of the Kurt Schwabe Institute for Measurement and Sensor Technology e.V. Meinsberg, adds, "At the same time, this work shows the tremendous potential in the development of miniaturized photonic analysis systems for medical diagnostics."
More information: Martin Fränzl et al. Thermophoretic trap for single amyloid fibril and protein aggregation studies, Nature Methods (2019). DOI: 10.1038/s41592-019-0451-6
Journal information: Nature Methods
Provided by Leipzig University