Credit: Natalia Deryugina/qimono
An international scientific group including a RUDN chemist have developed a complex copper-based compound with stronger antibacterial properties compared to analogs, and is cheaper to produce. The scientists confirmed that its effect is determined not by the substance's decomposition in the body, but by targeted influence on bacteria. The study was published in the Journal of Molecular Structure.
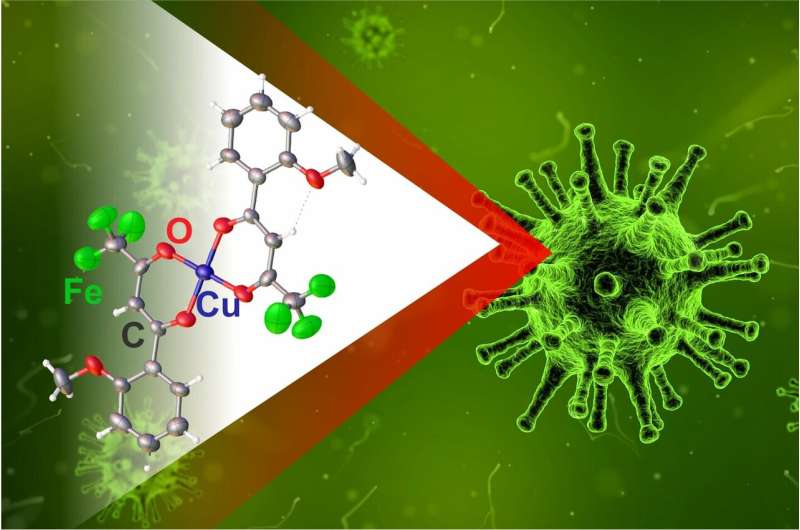
The team synthesized a biologically active compound using copper compounds and an organic substance. The result was a large molecule in which the copper atom was surrounded with a ligand—a part of the complex bound to the central atom. The size and physical characteristics of the ligand affected the properties of the whole molecule.
Combinations of transition metals and organic ligands play an important role in biological processes. They show a wide range of biological activities and are used in diagnostics and treatment of infectious, viral and oncological diseases, as well as metabolic disorders. Copper ions are important for aerobic microorganisms, plants and animals because they bind molecular oxygen in oxygen-transporting proteins and participate in electron transfer. Cupric copper complexes are considered prospective antimicrobial agents, a good alternative to platinum-based antitumor drugs, and potentially also modulators of inflammatory and autoimmune reactions.
"We've obtained several complexes of copper with 1,1,1-trifluoro-4- (2-methoxyphenyl)butan-2,4-dione and studied their biological activity against bacteria and fungi. The new complexes turned out to destroy bacteria better than pure ligand, but were less efficient against fungi," said Viktor Khrustalyov, a co-author of the work, Ph.D. in Chemistry, and the head of the Department of Inorganic Chemistry at RUDN.
The development of the substance required several stages. First, the researchers obtained the molecule of the ligand and diluted it in dimethylformamide (DMF), a solvent for polar compounds. After that, they added a copper-containing substance to the solution and obtained a complex compound. The copper atom played the role of a complexing agent, the central atom of the molecule around which ligands are placed. However, at this stage, the compound contained an extra solvent molecule. To finalize the product, the scientists tested two methods. First, they replaced DMF with dimethylsulfoxide (DSMO). The product was not soluble in it, and precipitated out. After that, the chemists isolated and purified the new substance. The second method consisted of two steps: removal of DMF and subsequent adding of DSMO. The structure of the substances was studied using infrared Fourier spectroscopy, elemental analysis, and X-ray structural analysis.
More information: Liliya A. Khamidullina et al. Synthesis, characterization, DFT calculations, and biological activity of copper(II) complexes with 1,1,1-trifluoro-4-(2-methoxyphenyl)butan-2,4-dione, Journal of Molecular Structure (2018). DOI: 10.1016/j.molstruc.2018.08.112
Provided by RUDN University