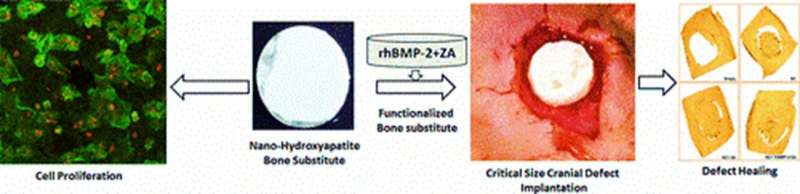
Illustration of the multifunctional materials experimentally developed in the study for in vivo applications of cell proliferation and cranioplasty. Defect healing in a rodent model is observed after 12 weeks of scaffold implantation at the site of defect with cell proliferation, radiography, micro-CT and histology analyses. Image credit: ACS Applied Materials & Interfaces. Credit: Multifunctional Materials, doi: https://doi.org/10.1088/2399-7532/aafc5b
Advances in materials science and production technology have enabled bone tissue engineering (BTE) strategies that generate complex scaffolds with controlled architecture for bone repair. The novel biomaterials can be further functionalized with bioactive molecules for biocompatibility by enhancing osteoinductivity (induce osteogenesis to initiate bone healing). In a recent study published in Multifunctional Materials, IOP Science, Arun Kumar Teotia and co-workers at the Departments of bioengineering, orthopedics, chemical engineering and biomedical engineering, in India, Finland and Sweden developed a novel, multifunctional, bilayered composite scaffold (BCS). The novel material contained ceramic nano-cement (NC) and the macroporous composite scaffold (CG) to mimic bone architecture during bone repair.
To functionalize the scaffolds, the materials scientists added recombinant human bone morphogenetic protein-2 (rhBMP-2) (BMP) and zoledronic acid (ZA). The scientists proposed that the composite scaffolds would support the proliferation of osteoblast progenitor cells, alongside the controlled release of loaded bioactive molecules to induce bone regeneration. Scientists in the same research team had previously developed a similar multifunctional material to test its initial impact during an in vivo pilot study.
In the present study, Teotia et al. observed a higher amount of mineralized tissue (MT) with functionalized scaffolds within 12 weeks of in vivo implantation in a larger group of rats with 8.5 mm critical cranial defects. The combined bilayered composite scaffolds (BCS) functionalized with zoledronic acid (ZA) (to form BCS+ZA) contained the highest MT deposition (13.9 mm3). Followed by the macroporous composite scaffold (CG) functionalized with BMP and ZA (CG+BMP+ZA) at 9.2 mm3 and BCS+ZA+BMP with 7.6 mm3 of MT deposition.
The MT values recorded in the study during bone regeneration were significantly higher than osteogenesis rates on the non-functionalized CG or BCS scaffolds alone (without bioactive molecules). The results supported the BTE strategies developed in the study to form an osteo-promotive multifunctional scaffold that could be implanted in vivo to repair critical defects.
A unique feature of bone tissue is its capacity to heal without scar formation as a highly dynamic tissue with substantial potential for regeneration. Natural bone formation occurs either via endochondral ossification within tubular bones (e.g. phalanges, femur) or during cartilage deposition, followed by ossification. In a third process, intramembranous direct ossification can occur in flat bones (skull, pelvis) without cartilage formation. Regeneration is a slow process in flat bones (skull, pelvis) due to limited mesenchymal stem cells (MSCs), requiring major cell recruitment from the periosteum or dura.
As a result, healing critical size defects in flat bones, such as the cranium is a challenge requiring optimized BTE strategies. Autograft bone flaps were preferred at first for cranioplasty to minimize immunological reactions, infections and foreign body recognition. Thereafter, scientists developed vascularized calvarium bone grafts as a preferred choice for cranial reconstruction in additional studies. However, the associated grafting strategies introduced complications during material resorption post-implantation and repair, alongside other clinical complications at the contact site between the implant and original bone. Regeneration and cell infiltration into a calvaria flap largely depends on progenitor cells that can migrate from the underlying dura or the overlying pericranial layers, to differentiate into active osteogenic cells for healing. If cell migration is occluded from the two membranes (dura and pericranium), bone formation would be significantly lower.
Scientists had already determined the two membranes to be important in playing a specific role during regeneration, although with age the role of periosteum in cranium regeneration is less significant. In the present study, Teotia et al. developed the hypothesis that an osteoconductive surface could maintain cross-talk between the dura and pericranial layers for early vascularization and clinical success. To accomplish this, they generated a bilayered scaffold architecture that integrated a resorbable biphasic nano-hydroxyapatite-calcium sulphate ceramic nano-cement (NC) in the upper layer and silk-bioglass-hydroxyapatite composite porous cryogel (CG) as an underlying layer.
Teotia et al. used the bilayered design to integrate the mechanical strength of NC as a protective upper layer and the porous composite CG layer as a surface for cell attachment, infiltration, proliferation and vascularization. The scientists expected the designed surfaces to maintain communication between the underlying dura and the overlying periosteal membranes. They functionalized the novel materials and implanted them in vivo in Wistar rats with critical cranial defects to evaluate the effect of bilayered porous architecture on osteoconduction and bone formation in preclinical, translational studies.
During materials fabrication, the scientists cast the NC into a concave-convex shaped architecture to match the shape of the cranium and allowed it to set, to engineer multifunctional bilayered scaffolds for cranioplasty. They formed circular BCS discs composed of upper NC and lower CG and conducted surgical procedures on the animal models. During surgery, Teotia et al. implanted the scaffold discs at the site of defect and performed ex vivo micro-CT and radiological analysis on the excised and harvested calvarium after sacrificing the animal models, 12 weeks after disc implantation.
The scientists completed radiological analyses of bone formation at the defect site to observe ossified tissue formation, using the nanoScan in vivo scanner for radiographical projections of the defect. They used micro-CT analysis to detect highly mineralized tissue (MT) formation and investigate defect filling in the 8.5 mm surgically induced circular defect (region of interest). By 12 weeks, mineralization did not achieve perfect closure in the animal model. The scientists used image quantifying software to show the highest amount of mineralized tissue formation in the BCS+ZA group, followed by the CG+ZA+BMP group, followed by CG+ZA+BMP and BCS+ZA+BMP groups.
Post-harvest, the scientists fixed the cranium samples for histology analysis and conducted hematoxylin and eosin (H&E) and Masson's trichrome staining of rat calvarias. They showed that both porous composite scaffold (CG) and the bilayered scaffold (NC+GC) (BCS) integrated well with existing bone at the site of the defect. The scaffolds provided porous surfaces for thorough cell infiltration. Teotia et al. also showed that functionalized scaffolds had consistently higher MT formation via histology assays due to the presence of osteoconductive and osteoinductive factors in the bioactive molecules composite compared to the non-functionalized groups. The histology results were consistent with the micro-CT results in the study.
In this way, Teotia et al. showed that multifunctional composite scaffolds could replace auto or allografts in large size, bone defects in the cranium. They showed that the multifunctional materials were able to induce early vascularization and enhance mineralization in vivo. As expected, the composite scaffolds allowed porous osteoconductive communication between early cell infiltration from the periosteum and the underlying dura layers during rapid bone formation. The multifunctional materials hold promise to enhance bone mineralization and early defect healing post-implantation. Teotia et al. propose to conduct additional studies in large pre-clinical animal models to optimize and translate the new biomaterial for clinical applications.
More information: Arun Kumar Teotia et al. Composite bilayered scaffolds with bio-functionalized ceramics for cranial bone defects: An in vivo evaluation, Multifunctional Materials (2019). DOI: 10.1088/2399-7532/aafc5b
Arun Kumar Teotia et al. Nano-Hydroxyapatite Bone Substitute Functionalized with Bone Active Molecules for Enhanced Cranial Bone Regeneration, ACS Applied Materials & Interfaces (2017). DOI: 10.1021/acsami.6b14782
Michael D. Hoffman et al. The effect of mesenchymal stem cells delivered via hydrogel-based tissue engineered periosteum on bone allograft healing, Biomaterials (2013). DOI: 10.1016/j.biomaterials.2013.08.005
Peter Frederik Horstmann et al. Composite Biomaterial as a Carrier for Bone-Active Substances for Metaphyseal Tibial Bone Defect Reconstruction in Rats, Tissue Engineering Part A (2017). DOI: 10.1089/ten.TEA.2017.0040
Journal information: ACS Applied Materials and Interfaces , Biomaterials
© 2019 Science X Network