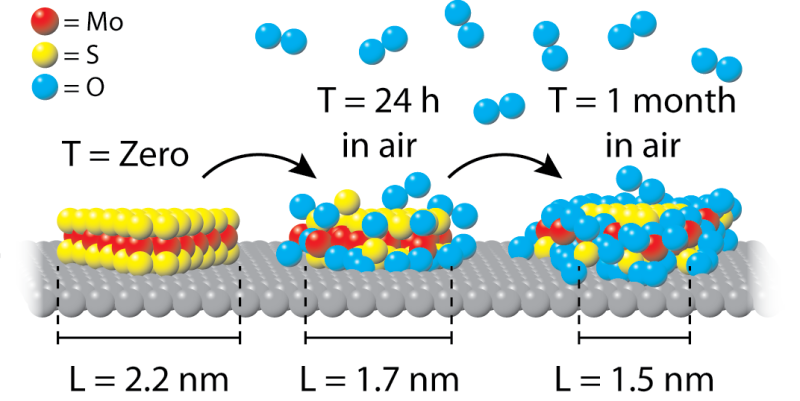
After 24 hours, a clean NiMoS2 particle (yellow-red) is already 20% covered with oxygen (blue spheres). In the subsequent period this process settles down, but a month later the coverage still has significantly increased. Credit: Leiden Institute of Physics
Catalyst research aims to make gasoline less polluting. It turns out that during experiments, it is actually necessary to protect catalysts from the air itself.
Thanks to catalysts, gasoline produces much less pollution today. Crude oil contains sulfur, which refineries filter out in the process of turning oil into gasoline. To do this, they add hydrogen and such catalysts as NiMoS2. The hydrogen removes a sulfur component of NiMoS2, giving the catalyst room to collect the sulfur from the oil.
Clean sample
To further improve the process, scientists research substances like NiMoS2. A small adaptation in the chemical composition could make it a more efficient catalyst. In such experiments, it is important to know how to keep the studied sample free from external influences.
Harmful air
A group of physicists led by Joost Frenken (Leiden University) and Patricia Kooyman (University of Cape Town) together with TU Eindhoven have now shown that exposure to air is very harmful. "Oxygen molecules from the air oxidize the NiMoS2 catalyst particles, so that further studying the sample essentially produces no relevant information," says first author Marien Bremmer. "We noticed that the oxidation occurred extremely fast at first, but slowed down in the long-term. This indicates the formation of a shielding oxide ring."
Credit: Leiden Institute of Physics
Publication
The research group used a high-resolution transmission electron microscope (HRTEM) to observe that after only 24 hours, 20 percent of each NiMoS2 particle is covered with oxygen. They describe the study in pubs.acs.org/doi/abs/10.1021/acs.jpcc.6b06030" rel="nofollow" target="_blank">the Journal of Physical Chemistry C.
More information: G. Marien Bremmer et al. Instability of NiMoSand CoMoSHydrodesulfurization Catalysts at Ambient Conditions: A Quasi in Situ High-Resolution Transmission Electron Microscopy and X-ray Photoelectron Spectroscopy Study, The Journal of Physical Chemistry C (2016). DOI: 10.1021/acs.jpcc.6b06030
Journal information: Journal of Physical Chemistry C
Provided by Leiden Institute of Physics