Demand for high-performance energy storage device is booming. There is a need for high-energy-density, rechargeable batteries, as electric vehicles, portable electronics, and sustainable energy harvesting are on the rise. Conventional lithium-ion (Li-ion) batteries are mature but limited in energy density, and don't satisfy the increasing demand for superior energy storage.
Lithium−sulfur (Li−S) batteries, with three to five times higher energy density (~2600 Wh kg-1 in theory) than Li-ion batteries, are a promising candidate. "The tremendously high energy density of Li−S batteries is because of the unique reaction mechanism. The transformation between sulfur and lithium sulfide involves a phase transition, giving an extremely high capacity compared to the intercalation mechanism," says Dr. Qiang Zhang, a professor at the Department of Chemical Engineering, Tsinghua University, China. "In commonly used aprotic electrolytes, the lithiation of sulfur is composed of a solid−liquid−solid conversion. The soluble intermediates, also known as lithium polysulfides, smooth the redox process and enable high cathode capacity."
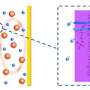
Unfortunately, soluble polysulfide intermediate is both a blessing and a curse. While contributing to the overall capacity, the solubility of polysulfides is accompanied with diffusivity, resulting in irreversible loss of active sulfur into electrolyte, anode or dead volumes.
"Capacity decay caused by polysulfides' detachment from the cathode framework has been a major issue obstructing the broad application of Li−S batteries. The usual solution to resolve this problem to date is to suppress polysulfide diffusion, such as adopting functional interlayers, anode-protecting additives, and novel electrolyte configurations," says Zhe Yuan, the first author of this work. "Nonetheless, the overflow of polysulfides in electrolyte should not be only imputed to their inevitable diffusion. The slow redox reaction rate of polysulfide intermediates is also to blame."
Zhang and his coworkers found an intriguing analogy of polysulfide overflow—an actual flood— and got inspired. "Compared to blocking the deluge with embankments, digging and enlarging canals or channels are seemingly more effective approaches in mitigating flooding," says Qiang. "Similarly, expediting polysulfide redox reaction, which is originally sluggish, removes the barrier of polysulfide consumption, and therefore relieves detrimental effects induced by polysulfide accumulation in the electrolyte."
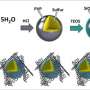
The team discovered that it was the incompatibility between polar lithium polysulfide molecules and commonly used nanocarbon cathode scaffolds that restrained the redox reactivity. Nanocarbon materials are excellent for Li−S batteries since they are highly conductive and porous. But their non-polar surface characteristic does not favor being attached by heteropolar polysulfides, "In that sense, we speculated that it would be advantageous to add a polar substance with high affinity for polysulfides into the cathode framework, and it turned out to be true," says Zhe.
The magical additive is cobalt disulfide (CoS2), a half-metallic, earth-abundant mineral. The team imported CoS2 into graphene frameworks by facile mechanical mixing. The modified cathodes armed with enhanced interactions between CoS2 and lithium polysulfides exhibited substantially accelerated polysulfide redox reactions, promoted energy efficiencies and elevated discharge capacities, as reported in Nano Letters.
More information: Zhe Yuan et al. Powering Lithium–Sulfur Battery Performance by Propelling Polysulfide Redox at Sulfiphilic Hosts, Nano Letters (2016). DOI: 10.1021/acs.nanolett.5b04166
Journal information: Nano Letters
Provided by Tsinghua University