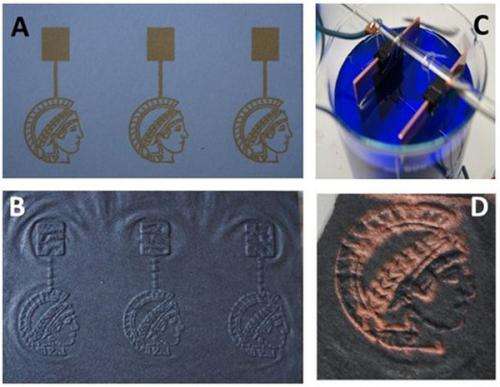
Electrifying Minerva: With the aid of a catalyst, which can be plotted on paper using an inkjet printer, the Potsdam-based researchers converted the symbol of the Max Planck Society into conductive graphite. Amorphous carbon is formed from the non-printed areas of the paper. The scientists then electrolytically coated the graphite Minerva with copper. Credit: MPI of Colloids and Interfaces
(Phys.org) —Paper is becoming a high-tech material. Researchers at the Max Planck Institute of Colloids and Interfaces in Potsdam-Golm have created targeted conductive structures on paper using a method that is quite simple: with a conventional inkjet printer, they printed a catalyst on a sheet of paper and then heated it. The printed areas on the paper were thereby converted into conductive graphite. Being an inexpensive, light and flexible raw material, paper is therefore highly suitable for electronic components in everyday objects.
Cost-efficient and flexible microchips are opening up applications in the electronics sector for which silicon chips are too expensive or difficult to make, and for which RFID chips, now available on a widespread basis, simply do not suffice: clothes, for instance, that monitor bodily functions, flexible screens, or labels that give more information about a product then can be printed on the packaging.
Although many scientists around the world are successfully developing flexible chips, they have been forced to almost always rely on plastics as the carrier and, in some cases, use polymers and other organic molecules as conductive components. These materials may meet many requirements; however, they are all, without exception, sensitive to heat. "Their processing cannot be integrated into the usual production of electronics, because temperatures in production can reach over 400 degrees Celsius," says Cristina Giordano, who leads a working group at the Max Planck Institute of Colloids and Interfaces and as now come up with an alternative solution.
Paper electronics enables three-dimensional conductive structures
Carbon electronics, which Giordano and her colleagues create from paper, can withstand temperatures of around 800 degrees Celsius during production in an oxygen-free environment, and would not have a negative impact on established processes. And that is not the only trump card of paper-based electronics. The light and inexpensive material can be processed very easily, even into three-dimensional conductive structures.
A simple path to electronics: First, an inkjet printer prints a catalyst in a certain pattern on a sheet of paper (A). Heat then converts the printed areas into conductive graphite in a nitrogen atmosphere and the non-printed areas are converted into amorphous carbon (B). The fact that only the printed areas are conductive is demonstrated by the subsequent process of electrolysis (C), whereby the conductive structure serves as a cathode and only the pattern treated with a catalyst is coated with copper. Credit: MPI of Colloids and Interfaces
The Potsdam-based researchers convert the cellulose of the paper into graphite with iron nitrate serving as the catalyst. "Using a commercial inkjet printer, we print a solution of the catalyst in a fine pattern on a sheet of paper," says Stefan Glatzel, who is responsible for bringing electronics to paper in his doctoral thesis. If the researchers then heat the sheets that were printed with a catalyst to 800 degrees Celsius in a nitrogen atmosphere, the cellulose will continue to release water until all that remains is pure carbon. Whereas an electrically conducting mixture of regularly structured carbon sheets of graphite and iron carbide forms in the printed areas, the non-printed areas are left behind as carbon without a regular structure, and they are less conductive.
That actual, precisely formed conducting paths are created in this way was demonstrated by the researchers in a simple experiment: First, they printed the catalyst on a sheet of paper in the pattern of Minerva, the subtle symbol of the Max Planck Society. The printed pattern was then converted into graphite. They then used the graphite Minerva as a cathode, which was electrolytically coated with copper. The metal was only deposited on the lines sketched by the printer.
An origami crane dressed in copper
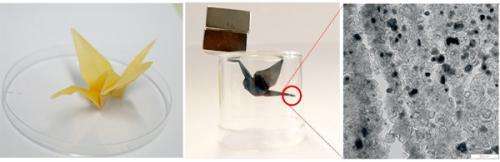
In another experiment, the team in Potsdam demonstrated how three-dimensional, conductive structures can be created using their method. For this experiment, the team folded a sheet of paper into an origami crane. This was then immersed in the catalyst and baked into graphite. "The three-dimensional form was completely retained, but consisted entirely of conductive carbon after the process," says Stefan Glatzel. He demonstrated this again by electrolytically coating the origami bird with copper. The entire crane subsequently had a copper sheen.
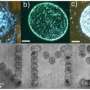
An origami figure takes flight: A crane made from folded paper is immersed in the ferric catalyst (left) by the Max Planck researchers in Potsdam. After the conversion, all that remains besides graphite is magnetic iron carbide, which allows the bird to fly towards the magnets (centre). The picture of a transmission electron microscope reveals the nanostructure of the carbon (right). Credit: MPI of Colloids and Interfaces
Finally, the actual process of the catalytic conversion was illustrated by the Max Planck scientists. Using a transmission electron microscope, they made a film of the process, observing how the catalyst journeyed through the paper in the form of nano droplets of an iron-carbon molten mixture, leaving graphite in its wake. This aspect, too, might be interesting for possible applications of the process. The better the understanding of chemists when it comes to what actually happens during the process, the better they can control the reaction. And this does not only apply to the production of paper electronics, but also to the manufacture of carbon nanotubes, where iron has been used as a catalyst for quite some time already.
Graphene structures from thin paper
This video of the graphite formation gave the researchers a comprehensive insight into catalytic conversion. Starting from these results, they are now trying to end a dispute over the mechanism behind the conversion. Some of their colleagues assume that the reaction takes place in a solid state. "Our study, however, shows that molten metal, or a so-called eutectic, is formed," says Giordano. "We observed something interesting here, as iron itself does not melt until temperatures of about 1500 degrees are reached."
Why the mixture of iron and carbon melts at relatively low temperatures is now being examined more closely by Giordano and her team. It may be possible to use this effect in other areas. Moreover, the researchers intend to further explore the potential of paper electronics. Here, they do not just want to exploit the magnetic properties of the material, which are a result of the iron carbide. By reducing the paper strength and subtly controlling the process, they also want to create conducting paths from graphene; by "graphene", they are referring to one of the carbon sheets that are stacked on top of each other in the graphite. "We will also combine graphite with other materials," explains Giordano. The inkjet printer makes this possible – it is from the printer's cartridges that iron nitrate solutions, as well as solutions from other metal salts or dispersions containing metal particles finely distributed in water can be brought to paper.
More information: Glatzel, S., Schnepp, Z. and Giordano, C. From Paper to Structured Carbon Electrodes by Inkjet Printing, Angewandte Chemie International Edition, published online, January 17, 2013; DOI: 10.1002/anie.201207693
Journal information: Angewandte Chemie International Edition
Provided by Max Planck Society


.jpg)