The molecular mechanisms of GlyT1 in physiology and pharmacology. Credit: Zhao Yan's group
Schizophrenia is a highly disabling mental disorder, and numerous studies have shown that the hypofunction of the N-methyl-d-aspartate (NMDA) receptor is one of its pathogenic mechanisms. Glycine transporter 1 (GlyT1), a glycine transporter protein, is highly co-localized with the NMDA receptor. Inhibition of GlyT1 can increase the concentration of glycine in the synaptic cleft, thereby indirectly promoting NMDA receptor activation. Therefore, GlyT1 is considered a key target for the treatment of schizophrenia.
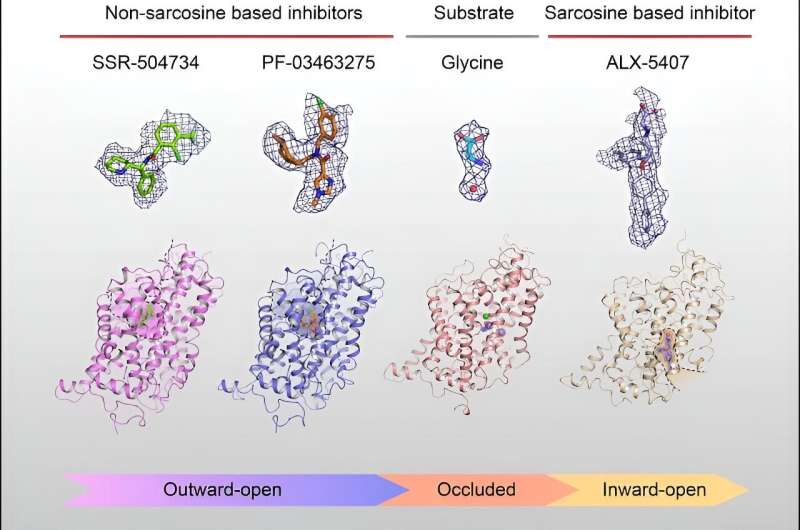
In a study published in Cell a research team led by Prof. Zhao Yan from the Institute of Biophysics of the Chinese Academy of Sciences reveals three different conformations of the full-length wild-type human GlyT1 transporter, providing the elucidation of substrate recognition and the mechanism by which three anti-schizophrenia drug candidates selectively inhibit GlyT1.
The researchers report the structure of GlyT1 with glycine bound in an occluded state, while identifying the binding sites of a chloride ion and two sodium ions that were co-transported with glycine, elucidating the coupling mechanism of substrate and ion binding during transport.
Currently, clinical candidate drugs targeting GlyT1 for the treatment of schizophrenia can be divided into sarcosine-based and non-sarcosine-based classes. The researchers found that the initial lead sarcosine-based inhibitor, ALX-5407, binds to an inward pocket of GlyT1.
They also identified the first patented non-sarcosine-based inhibitor SSR504734, and the drug PF-03463275, which is currently in Phase II clinical trials, to bind to an outward pocket of GlyT1.
This study explores the substrate recognition, ion binding, conformational transition of GlyT1, and the structure-activity relationships with the clinical trial drugs. The researchers believe it will help accelerate the drug development process targeting GlyT1, providing strong theoretical support for the design and development of anti-schizophrenia drugs.
More information: Yiqing Wei et al, Transport mechanism and pharmacology of the human GlyT1, Cell (2024). DOI: 10.1016/j.cell.2024.02.026
Journal information: Cell
Provided by Chinese Academy of Sciences