This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists reveal VMAT2 transport and inhibition mechanisms using cryo-EM
Neurotransmitters are a class of signaling chemicals, including monoamines such as serotonin, dopamine, and histamine, which play a vital role in a variety of neurological activities, including mood, memory, growth and development, and drug addiction. The cytosolic neurotransmitters in presynaptic neurons must be transported into synaptic vesicles for storage and subsequent release.
The packaging of monoamines into vesicles is mediated by vesicular monoamine transporter 2 (VMAT2). Importantly, several drugs targeting VMAT2 have been used to treat hypertension and hyperactivity disorders.
Human VAMT2 is a small membrane protein with a molecular weight of only 56 kDa, making it extremely difficult for cryo-EM analysis. Prof. Jiang Daohua's group from the Institute of Physics of the Chinese Academy of Sciences has overcome the challenge by screening fusion proteins, and they reconstructed the high-resolution structures of VMAT2 binding to three clinical drugs and the substrate serotonin.
Combined with functional experiments and molecular dynamic simulations, the researchers described the molecular mechanisms of substrate recognition and drug inhibition of VMAT2. The study, titled "Transport and inhibition mechanism of human VMAT2," was published in Nature.
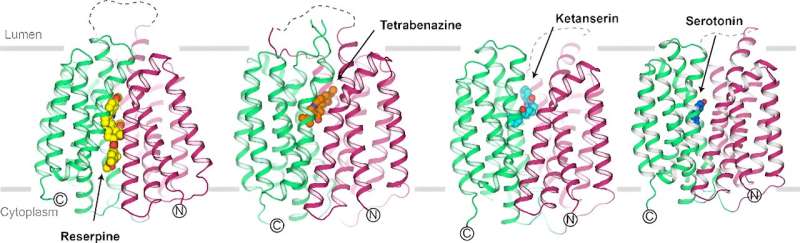
The cryo-EM structures were determined in cytoplasm facing, occluded and lumen facing states, representing three typical conformations in the transport cycle of VMAT2. The structures also revealed the inhibitory mechanisms of different drugs. For example, reserpine competes with serotonin for binding to the cytoplasm facing VMAT2, but tetrabenazine and ketanserin stabilize VMAT2 in occluded and lumen facing states, respectively.
In addition, the structures provide important insights into understanding the different pharmacological properties of reserpine, tetrabenazine and ketanserin. Furthermore, serotonin-bound VMAT2 adopts a lumen-facing conformation, a state that favors substrate release.
This study advances the comprehension of VMAT2 functions and facilitates the mechanistic understanding of substrate recognition, drug inhibition, and drug development of VMAT2. Meanwhile, the VMAT2 fusion protein strategy used in this study could be applied to other small membrane proteins, which will facilitate the structure analysis of membrane transporter proteins and other small proteins by cryo-EM.
More information: Di Wu et al, Transport and inhibition mechanisms of human VMAT2, Nature (2023). DOI: 10.1038/s41586-023-06926-4
Journal information: Nature
Provided by Chinese Academy of Sciences





















