This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Structural basis of bile salt extrusion and small-molecule inhibition in human BSEP
Bile salts play a crucial role in the digestion and absorption of dietary fats in the small intestine, particularly in the duodenum. The human bile salt export pump (BSEP), also known as ABCB11, mediates a variety of bile salts transport into canaliculi against a steep concentration gradient in the enterohepatic circulation. BSEP dysfunction, caused by mutations or induced by drugs, is frequently associated with severe cholestatic liver disease.
To date, several structures of BSEP under two conformations were reported, but it is unknown how the small molecules can inhibit BSEP function and how bile salts are extruded and released during the transport cycle.
The group of Prof. Kaspar Locher (IMBB, ETHZ), in collaboration with the groups of Prof. Henning Stahlberg (EFPL & University of Lausanne), reported three cryo-EM structures of human BSEP in lipidic nanodiscs in distinct conformations at a resolution 2.8–3.2 Å.
The research provides structural and functional insight into the mechanism of bile salt extrusion and into small-molecule inhibition of BSEP, which may rationalize drug-induced liver toxicity. The work is published in the journal Nature Communications.
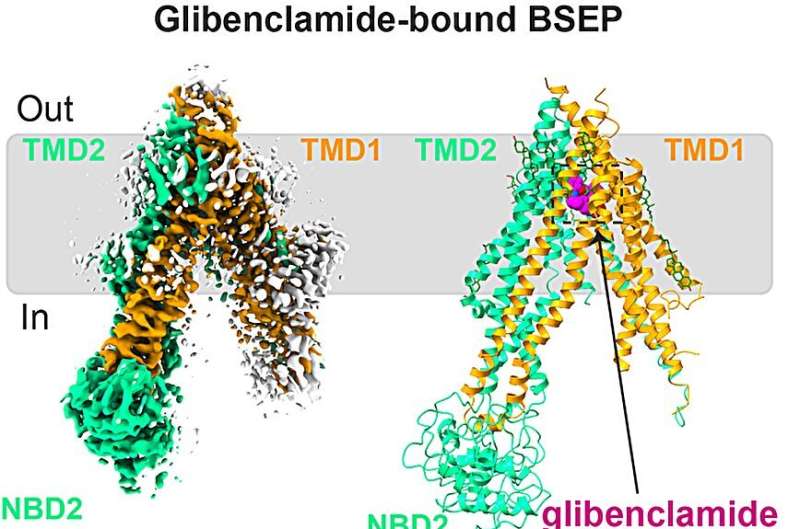
The researchers determined the structure of glibenclamide-bound human BSEP, revealing the basis of small-molecule inhibition. Glibenclamide binds the apex of a central binding pocket between the transmembrane domains, preventing BSEP from undergoing conformational changes, and thus rationalizing the reduced uptake of bile salts.
Two high-resolution structures of BSEP trapped in distinct nucleotide-bound states were reported by using a catalytically inactivated BSEP variant (BSEPE1244Q) to visualize a pre-hydrolysis state, and wild-type BSEP trapped by vanadate to visualize a post-hydrolysis state.
Their results shed light on the functional consequences and the molecular basis of BSEP mutations, providing insight into their pathogenetic mechanism. In conclusion, the research may help in the design of drugs targeting the liver while avoiding inhibition of BSEP and elucidate the structural insights of clinical mutations, especially in NBDs, which may help in the design of new correctors.
More information: Hongtao Liu et al, Structural basis of bile salt extrusion and small-molecule inhibition in human BSEP, Nature Communications (2023). DOI: 10.1038/s41467-023-43109-1
Provided by ETH Zurich