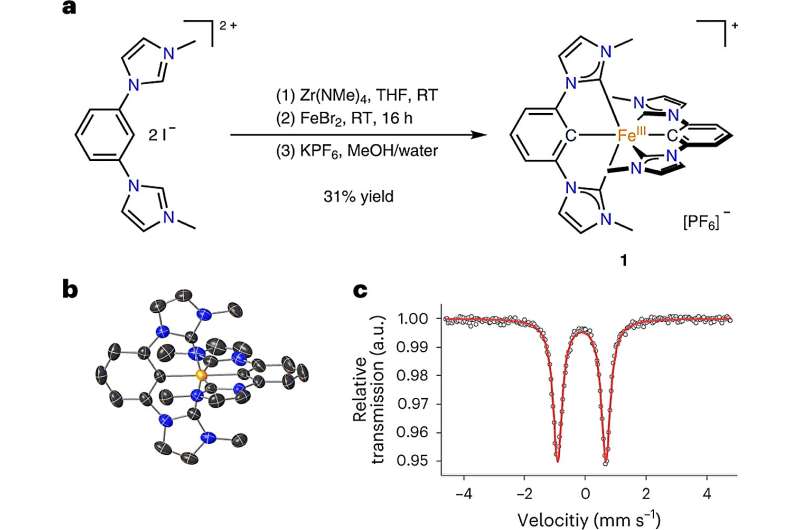
Synthesis, X-ray structure and Mössbauer spectrum of 1. a, Activation of the pro-ligand using a zirconium reagent with subsequent transmetalation onto iron. MeOH, methanol; RT, room temperature; THF, tetrahydrofuran. Due to the donor strength of the ligand, the FeII complex is oxidized under air to the FeIII complex 1. b, Structure of the cation of 1, as determined by X-ray diffraction. Hydrogen atoms and counter ion are omitted for clarity. c, Mössbauer spectrum of 1 at 80 K showing the characteristic doublet of a low-spin FeIII complex. Credit: Nature Chemistry (2023). DOI: 10.1038/s41557-023-01137-w
Scientists from Paderborn University, working under Professor Matthias Bauer, have achieved a breakthrough in the field of sustainable chemistry: together with a team of researchers from the universities of Rostock, Mainz, Göttingen, Innsbruck and Kassel, they have developed a chemical complex that converts light into energy for reactions and optical applications—in a sustainable way, since the material can be used to save huge amounts of CO2.
The new compound has potential applications in areas such as diodes, or in converting solar energy into chemical energy. The results have now been published in Nature Chemistry.
Saving carbon dioxide
"What makes this complex special is that unlike the systems currently in use, it contains iron as the central element," Bauer explains. Previously, precious-metal-based compounds were generally used for photochemical reactions and photophysical applications.
"However, manufacturing these produces carbon dioxide emissions of around 30 tons per kilogram. If precious metals are replaced with iron, the potential reduction in climate-damaging CO2 is huge," Bauer adds. By comparison, manufacturing a kilogram of iron only produces around two kilograms of the climate-damaging gas.
'Sustainability to the power of two'
"For the first time, the design of the compound being examined has enabled us to implement a property that is extremely rare in chemical compounds and unprecedented in iron compounds," explains Dr. Jakob Steube, one of the key members of Bauer's team. The complex glows in two different colors when exposed to light of a certain energy.
These photophysical properties will for example enable white light-emitting diodes to be made using iron compounds. In addition, the complex can be used to transform solar energy into chemical energy.
"We were able to demonstrate that following absorption with light, chemical reactions are possible with our new compound," Bauer explains. "This gives us sustainability to the power of two, namely energy conversion using a virtually CO2-neutral compound plus the combination of areas of application in photochemistry and photophysics. This can absolutely be described as a mini breakthrough."
These findings were gained as part of the priority program "Light-controlled reactivity of metal complexes." Headed by co-author Professor Katja Heinze of the University of Mainz, the program examines the question of how to ensure sustainable chemical reactions in the future despite increasingly scarce resources, as well as ways of using new energy sources such as sunlight.
More information: Jakob Steube et al, Janus-type emission from a cyclometalated iron(iii) complex, Nature Chemistry (2023). DOI: 10.1038/s41557-023-01137-w
Journal information: Nature Chemistry
Provided by Universität Paderborn