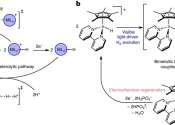
Exploring the potential of single-atom catalysts
There is a high level of interest, even excitement, among chemists and materials scientists about the potential of single-atom catalysts (SACs), but their development relies on very specialized tools available only at synchrotrons ...