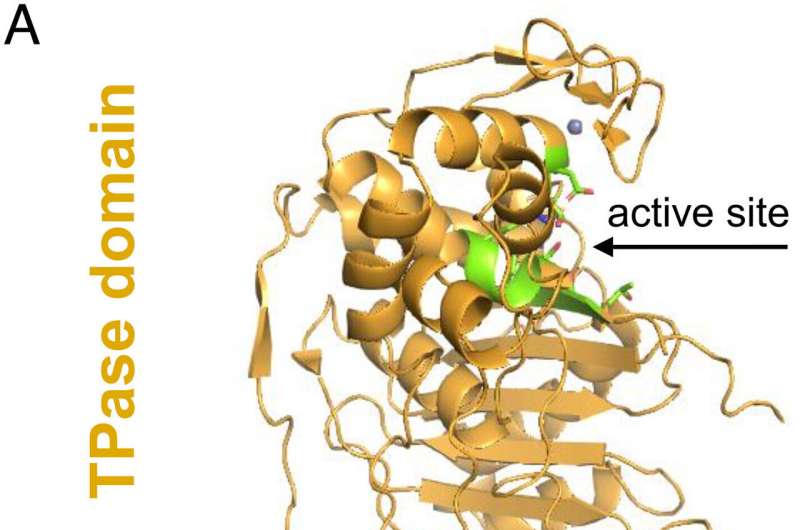
(A) Domain organization in the structure of A. baumannii PBP2. The pedestal domain consists of anchor (red), linker (cyan), and head (purple) subdomains and is located at the N terminus. The TPase domain (gold) is located at the C terminus and contains the catalytic site (green). (B) Close-up view of the TPase active site of PBP2. The catalytic site contains three conserved motifs (green), and the nearby Zn (gray sphere) is also shown. Credit: Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2215237120
A new study has shown that zinc plays a key role in a hospital superbug that doctors struggle to treat due to its resistance to antibiotics.
Acinetobacter baumannii (AB) infections can cause infections in the blood, urinary tract, and lungs or in wounds in other parts of the body and are a particular issue in hospital situations. Patients can also carry AB without presenting symptoms or becoming unwell, while the bacteria causes other people to become very sick or even to die.
Unfortunately, many AB infections are resistant to antibiotics, which makes them difficult to treat.
Now, new research, published today in Proceedings of the National Academy of Sciences (PNAS), has found that zinc plays a key role in how AB cells make their shape. This identifies it as a new target to treat infections.
Just as the discovery of antibiotics was a happy accident, Dr. Carmina Micelli noticed the importance of zinc when she was investigating new drug targets by studying the function of penicillin-binding proteins (PBPs), a group of chemicals known as enzymes, which control cell shape in AB bacteria.
She said, "I was using X-ray crystallography to study PBPs, when I noticed a single zinc ion in a particular structural part of PBP2. I knew this hadn't been discovered before."
"When zinc was removed the stability of PBP2 was reduced and resulted in loss of its function. This caused AB to change shape, in the same way that antibiotics would."
Dr. Micelli worked with researchers at Northeastern and Tufts universities in the US, to show that such cells were also more vulnerable to exposure to existing penicillin-based drugs.
"This study shows that AB cell shape appears to require zinc to function. Maintenance of cell shape is vitally important for bacterial life and the enzyme PBP2 is at the heart of this process," added Dr. Micelli.
Professor David Roper for the School of Life Sciences at Warwick said, "There are several non-antibiotic drugs that can be used to trap zinc already under analysis for use in medicine. Combining these drugs with certain penicillin-based compounds could provide a new way to deal with AB infections and combat highly resistant forms of this bacteria."
"This research is also related to similar observations in Clostridium difficile (C.diff)—a common bacterial infection causing diarrhea—so our work could have even wider medical application."
More information: Carmina Micelli et al, A conserved zinc-binding site in Acinetobacter baumannii PBP2 required for elongasome-directed bacterial cell shape, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2215237120
Journal information: Proceedings of the National Academy of Sciences
Provided by University of Warwick