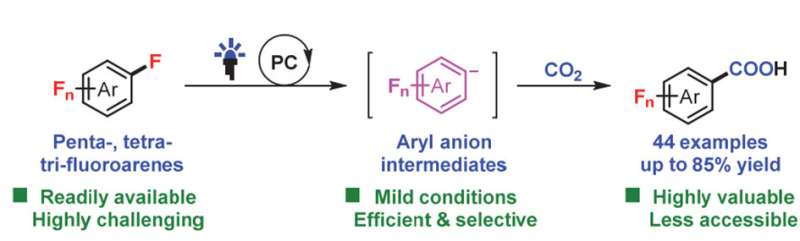
The first visible-light photoredox-catalyzed selective carboxylation of C(sp2)−F bonds in polyfluoroarenes with CO2 is developed, which would provide a facile and efficient way to construct polyfluoroaryl carboxylic acids, the privileged scaffolds in pharmaceuticals, agrochemicals and optoelectronics. Polyfluoroaryl anions are generated from polyfluoroaryl radical and may serve as the possible key intermediates, which may open a new avenue for photocatalytic transformation of inert aryl C(sp2)−F bonds. Credit: Chinese Journal of Catalysis (2022). DOI: 10.1016/S1872-2067(22)64140-8
Carbon dioxide (CO2) is a notorious greenhouse gas and also a promising building block in synthetic chemistry due to its abundance, easy availability, nontoxicity, and recyclability. However, the inert nature of CO2 has a set barrier for its efficient chemical transformation. Recently, photochemistry has provided a sustainable, benign, and powerful way for carboxylation with CO2 under mild conditions by using photons as energy source.
In particular, the visible-light driven carboxylation of aryl halides with CO2 is attractive due to the importance of aryl carboxylic acid derivatives and the easy access of diverse aryl (pseudo)halides. In contrast to traditional methods where reactive but metallic reductants or sacrificial electrodes are always involved, the photocatalytic process features mild conditions with mild electron donors.
Iwasawa, König, Jana and others contributed significantly to this field by realizing carboxylation of aryl carbon-heteroatom bonds in various aryl (pseudo)halides via photoredox/transition metal dual catalysis. Despite this great achievement, there is no report of visible-light photoredox-catalyzed carboxylation of aryl C−F bonds with CO2. Thus, the development of visible-light photocatalytic carboxylation of aryl C−F bonds with CO2 with high selectivity is urgent but extremely challenging.
Fluorine chemistry has important applications. C−F bond functionalization is important to convert readily available polyfluoroarenes to partially fluorinated compounds, which are highly valuable but less accessible. Polyfluoroaryl carboxylic acids and derivatives are important structures in pharmaceuticals, agricultures, and organic functional materials.
Constructing polyfluoroaryl carboxylic acids with green CO2 under mild conditions via photocatalysis is promising. However, such a strategy may face great challenges. First, aryl C−F bonds are very strong, with set barriers for efficient cleavage. Second, the chemical inertness of CO2 makes it difficult for efficient cross coupling with C−F bonds. Finally, photocatalysis would introduce instable and untamable radical intermediates which usually cause diverse side reactions that are hard to suppress.
Recently, a research group led by Prof. Da-Gang Yu from Sichuan University, China reported the first visible-light photoredox-catalyzed selective carboxylation of C(sp2)−F bonds in polyfluoroarenes with CO2. Such work features wide and challenging substrate scope including penta-, tetra- and trifluorinated arenes with moderate to high yields. What's more, such transformation can pick up one particular C(sp2)−F bond from the others with benign selectivity.
The results were published in the Chinese Journal of Catalysis.
More information: Zhi-Yu Bo et al, Visible-light photoredox-catalyzed selective carboxylation of C(sp2)−F bonds in polyfluoroarenes with CO2, Chinese Journal of Catalysis (2022). DOI: 10.1016/S1872-2067(22)64140-8
Provided by Chinese Academy of Sciences