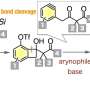
Aryl radical formation by aryl halide bond cleavage by a N-heterocyclic carbene catalyst
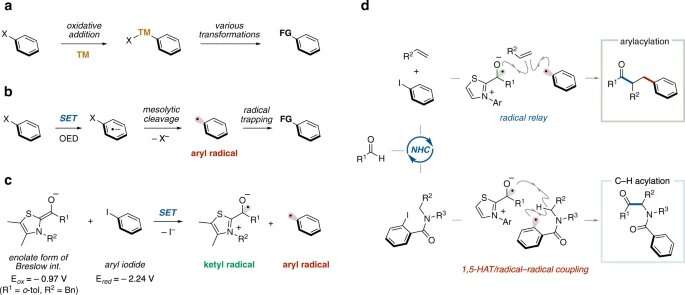
Aryl halides with a benzene ring directly bonded to a halogen atom are readily available and chemically stable, so they are used as a source of benzene rings in organic synthesis. For example, a chemical reaction that generates a highly reactive aryl radical from an aryl halide using a toxic tin compound has long been known as a method for supplying a benzene ring. In recent years, chemical reactions have been developed in which an aryl halide is reduced using a metal catalyst or a photocatalyst followed by cleavage of the bond between the benzene ring and the halogen atom to generate an aryl radical. However, since the methods previously reported require metal salts and/or excess amounts of an oxidizing agent or a reducing agent, chemical reactions with less environmental impact are desirable.
The research group of Kanazawa University led by Prof. Ohmiya has been developing novel chemical reactions using newly developed metal-free organic catalysts to produce various useful chemicals in a much easier manner than with conventional methods. In the present study, the group succeeded in generating aryl radicals from aryl iodides, a type of aryl halide, under mild conditions without the need for light or metal salts, using an N-heterocyclic carbene catalyst and the aryl radicals thus formed were used for organic syntheses.
A single electron transfer from an enolate intermediate consisting of a thiazolium-type N-heterocyclic carbene catalyst and an aldehyde to an aryl iodide and the subsequent cleavage of the bond between the benzene ring and the iodine atom generate an aryl radical in a catalytic manner. Considering the oxidation potential of the enolate intermediate (Eox = -0.97 V) and the reduction potential of the aryl iodide (Ered = -2.24 V), single electron transfer from the enolate intermediate to the aryl iodide, i.e. single electron reduction, is thermodynamically unfavorable. However, it is considered that the reaction took place due to kinetic factors because the two reaction steps, i.e. 1) single electron transfer from the enolate intermediate to the aryl iodide and 2) cleavage of the bond between the benzene ring and the iodine atom, proceed rapidly. The aryl radical generated acts as a source of the benzene ring, the difunctionalization of the alkene proceeds, and a benzene-ring substituted ketone is obtained. In addition, by using the aryl radical generated for the intramolecular hydrogen abstraction reaction, the dehydrogenative acylation of the amide proceeds, and an α-aminoketone compound can be obtained. Substrates with various functional groups can be used in these molecular conversion reactions. Derivatives of pharmaceuticals can also be synthesized by the dehydrogenative acylation of amides.
The results of this study are the development of a chemical reaction that cleaves the bond between the benzene ring of an aryl halide and the halogen atom by using an organic catalyst that has a low impact on the environment, leading to generation of an aryl radical. Since aryl radicals can be easily generated from aryl halides that are widely used in organic synthesis, this is expected to be a powerful technology for precisely synthesizing medical and agricultural drugs as well as chemical materials.
More information: Yuki Matsuki et al, Aryl radical-mediated N-heterocyclic carbene catalysis, Nature Communications (2021). DOI: 10.1038/s41467-021-24144-2
Journal information: Nature Communications
Provided by Kanazawa University