Visible-light-driven arylcarboxylation of styrenes with carbon dioxide and aryl halides
The use of carbon dioxide (CO2), which is an ideal one-carbon (C1) building block and is sustainable, abundant, low-cost and nontoxic, has attracted great attention in fine chemical synthesis. However, traditional CO2 fixation usually suffers from high temperature, high pressure of CO2 and the use of strong base.
Visible-light-driven photoredox catalysis (PRC) for CO2 use is an environmentally friendly process in organic synthesis of complex molecules.
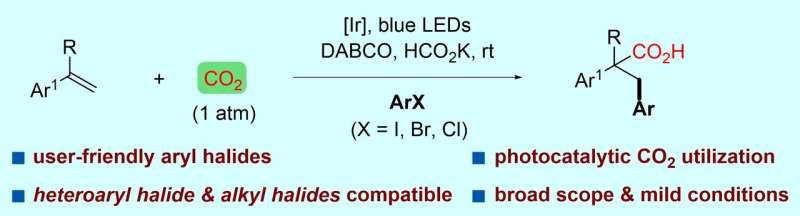
In a study published in the Journal of the American Chemical Society, a group led by Prof. LI Gang at Fujian Institute of Research on the Structure of Matter of the Chinese Academy of Sciences reported an interesting protocol of visible-light-driven reductive arylcarboxylation of styrene derivatives with CO2 (1 atm) and aryl halides.
This is the first example of the visible-light-driven Meerwein-arylation-type difunctionalization of alkenes using aryl halides, which are often bench-stable, inexpensive and widely available.
The researchers found that a wide range of aryl iodides and bromides were viable with this reaction to produce arylcarboxylation products of various styrene derivatives. Besides, pyridyl halides, alkyl halides and electron-deficient aryl chlorides that are challenging to reduce were also compatible with this reaction.
In the preliminary mechanistic study, the control experiments without using aryl halides could afforded a decarboxylation product, suggesting that a highly reductive CO2 radical anion might be involved in this reaction to reduce aryl halides to generate aryl radicals. When a "radical clock" substrate was used, ring-opening product was produced, indicating a benzyl radical might be involved. Besides, the reaction could be scalable without significant decrease in the yield of desired products.
This study may provide a new opportunity for exploring novel visible-light-driven Meerwein-type arylation-addition reactions employing user-friendly aryl halides as the radical sources, as well as developing new photocatalytic use reactions of CO2.
More information: Hao Wang et al. Visible-Light-Driven Reductive Carboarylation of Styrenes with CO2 and Aryl Halides, Journal of the American Chemical Society (2020). DOI: 10.1021/jacs.0c03144
Journal information: Journal of the American Chemical Society
Provided by Chinese Academy of Sciences