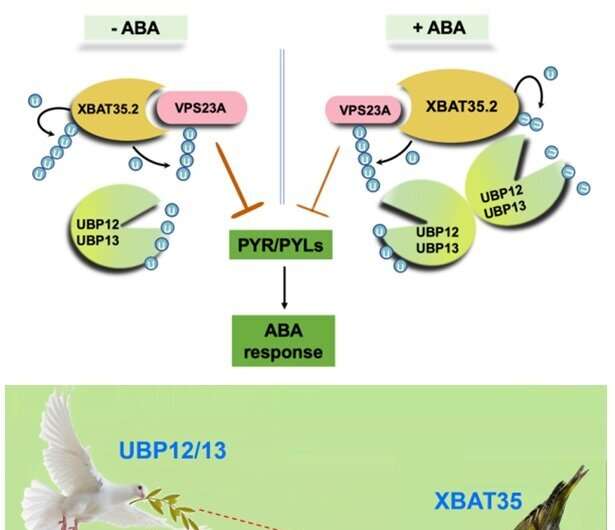
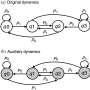
The model of UPB12/13-XBAT35-VPS23A-PYR action in ABA perception. Credit: IGDB
When plants are under drought stress, a large amount of the stress hormone abscisic acid (ABA) is produced to help them resist drought stress. In recent years, studies have found that ubiquitination mediated protein degradation plays an important role in regulating the ABA signaling pathway. Vacuolar protein sorting 23A (VPS23A), a key component of the endosomal sorting complex required for transport I, mediates the entry of ubiquitinated ABA receptors into the vacuole for degradation. The E3 ubiquitin ligase XBAT35.2 can ubiquitinate and modify VPS23A to promote its entry into the 26S proteasome for degradation, thereby positively regulating the ABA signaling pathway.
Ubiquitination of substrate proteins can determine their protein activity, subcellular localization and even protein stability. Therefore, the level of ubiquitination of proteins must be finely regulated. So what is the mechanism that plants adopt to maintain an appropriate ubiquitination level of key proteins?
To explore this question, researchers from XIE Qi's team at the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences found deubiquitinases ubiquitin-specific protease 12 (UBP12) and UBP13 are the interacting proteins of VPS23A via performing a screening assay. A series of biochemical experiments demonstrated that UBP12 and UBP13 can deubiquitinate VPS23A and maintain the protein stability of VPS23A.
They further analyzed the responses of UBP12 and UBP13 mutants to ABA and drought stress, and found that the mutants of UBP12 and UBP13 are more sensitive to ABA treatment and are insensitive to drought stress compared with the wild type plants.
Subsequent genetic experiments demonstrated that UBP12 and UBP13 can participate in the regulation of ABA signaling and plant responses to drought stress through VPS23A. Therefore, the E3 ubiquitin ligase XBAT35.2 and the deubiquitinating enzymes UBP12 and UBP13 act as accelerators and brakes that balance VPS23A ubiquitination homeostasis at different environments.
Unexpectedly, in addition to VPS23A, the researchers further found that the E3 ubiquitin ligase XBAT35.2 of VPS23A is also a substrate of UBP12 and UBP13. UBP12 and UBP13 can deubiquitinate and stabilize XBAT35.2 under ABA treatment. Hence, even though UBP12 and UBP13 stabilize VPS23A, they could also promote the degradation of VPS23A under ABA treatment. Altogether, UBP12 and UBP13 are versatile deubiquitinases in ABA signaling.
Global climate change has led to frequent occurrence of extreme weather, posing a huge threat to agricultural production. As an important abiotic adversity, drought brings immeasurable disasters to the world's agriculture every year. Therefore, studying the plant response mechanism to drought and mining important drought resistance genes play crucial roles in the sustainable development of agriculture.
This study is published in Science Advances.
More information: Guangchao Liu et al, The deubiquitinases UBP12 and UBP13 integrate with the E3 ubiquitin ligase XBAT35.2 to modulate VPS23A stability in ABA signaling, Science Advances (2022). DOI: 10.1126/sciadv.abl5765
Journal information: Science Advances
Provided by Chinese Academy of Sciences