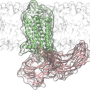
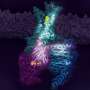
Conformational changes of βarr1 upon binding to phosphorylated β2V2R in rHDLs bound to the full agonist. a Structural differences of βarr1 between the basal state (gray, PDB ID: 1G4M) and in complex with β1AR-V2R6P and Fab30 (purple, PDB ID: 6TKO). The structural model was prepared with Cuemol (http://www.cuemol.org/). b 1H-13C HMQC spectra of [u-2H, Ileδ1-13C1H3] βarr1 in the basal state (left, black), the complex with phosphorylated β2V2R in rHDLs bound to the full agonist (middle, red), and the complex with both phosphorylated β2V2R in rHDLs bound to the full agonist and Fab30 (right, purple). c Normalized chemical shift differences between the basal state and the complex with phosphorylated β2V2R in rHDLs bound to the full agonist (left), and those between the complex with phosphorylated β2V2R in rHDLs bound to the full agonist and the complex with both phosphorylated β2V2R in rHDLs bound to the full agonist and Fab30 (right). Asterisks indicate residues with resonances broadened beyond detection upon the addition of phosphorylated β2V2R in rHDLs bound to the full agonist (left) and those upon the addition of Fab30 (right). N.D. indicates residues with resonances that were not observed both before (B; middle) and after (B; right) Fab30 addition. The error bars were calculated based on the digital resolution of the spectra, as described in “Methods”. d Distribution of the isoleucine residues on the structure of βarr1 in the basal state (PDB ID: 1G4M). The residues with resonances exhibiting chemical shifts larger than 0.1 ppm upon binding to phosphorylated β2V2R in rHDLs bound to the full agonist are shown as red spheres. Credit: Nature Communications (2021). DOI: 10.1038/s41467-021-27482-3
In a structural analysis that could help inform efforts to develop new drugs for treating a myriad of diseases, RIKEN biologists have shed light on how the interaction between key signaling proteins and their regulatory partners controls a wide variety of processes within the cell.
Signaling proteins known as G-protein-coupled receptors (GPCRs) are found in the membranes of cells, and they convert signals from outside a cell into responses within a cell. They are responsible for reacting to signals from hormones, neurotransmitters and sensory signals from the eyes, nose and mouth. The critical role they play in cell biology is highlighted by the fact that roughly half of all drugs on pharmacy shelves target GPCRs.
There has been much research into how GPCRs activate their scaffolding partners, known as β-arrestins, by binding to them. For a long time, β-arrestins were thought to be activated only by the tail end of GPCRs. But now, Ichio Shimada and Yutaro Shiraishi at the RIKEN Center for Biosystems Dynamics Research and five collaborators have shown how activation of β-arrestin also involves engagement with the GPCR core.
Using nuclear magnetic resonance spectroscopy, the researchers discovered that β-arrestins bound to the tail of an interacting GPCR are only partially activated—subsequent binding with the core region is needed to push the β-arrestins into full activation mode. This, in turn, triggers downstream signaling cascades involved in everything from sight and smell to immune regulation and neuronal communication.
"We have demonstrated that β-arrestin activation by GPCR involves two phases," says Shimada. That two-part chain of events helps to stabilize β-arrestins in their fully engaged, activated conformation.
With an eye to drug development, the researchers also detailed how a drug-like antibody fragment helped to shift the balance in structural conformations to promote more activation-inducing interactions between β-arrestins and GPCRs, even in the absence of core binding.
That finding highlights a path forward for therapeutically biasing the signaling activities of β-arrestins. "Variants of the antibody may be good candidates to modulate the signal transduction mediated by the GPCR–arrestin complex," says Shiraishi.
The findings also underscored the conformational agility of β-arrestins interacting with GPCRs—and the mechanistic relevance of this structural flexibility. "The plasticity of β-arrestin in complex with a GPCR may explain its multifunctionality," says Shimada.
Looking ahead, the ability of these signal transduction proteins to serve so many roles in the cell may be driven by additional interactions with GPCRs yet to be discovered, Shimada adds.
More information: Yutaro Shiraishi et al, Biphasic activation of β-arrestin 1 upon interaction with a GPCR revealed by methyl-TROSY NMR, Nature Communications (2021). DOI: 10.1038/s41467-021-27482-3
Journal information: Nature Communications
Provided by RIKEN
![Conformational changes of βarr1 upon binding to phosphorylated β2V2R in rHDLs bound to the full agonist. a Structural differences of βarr1 between the basal state (gray, PDB ID: 1G4M) and in complex with β1AR-V2R6P and Fab30 (purple, PDB ID: 6TKO). The structural model was prepared with Cuemol (http://www.cuemol.org/). b 1H-13C HMQC spectra of [u-2H, Ileδ1-13C1H3] βarr1 in the basal state (left, black), the complex with phosphorylated β2V2R in rHDLs bound to the full agonist (middle, red), and the complex with both phosphorylated β2V2R in rHDLs bound to the full agonist and Fab30 (right, purple). c Normalized chemical shift differences between the basal state and the complex with phosphorylated β2V2R in rHDLs bound to the full agonist (left), and those between the complex with phosphorylated β2V2R in rHDLs bound to the full agonist and the complex with both phosphorylated β2V2R in rHDLs bound to the full agonist and Fab30 (right). Asterisks indicate residues with resonances broadened beyond detection upon the addition of phosphorylated β2V2R in rHDLs bound to the full agonist (left) and those upon the addition of Fab30 (right). N.D. indicates residues with resonances that were not observed both before (B; middle) and after (B; right) Fab30 addition. The error bars were calculated based on the digital resolution of the spectra, as described in “Methods”. d Distribution of the isoleucine residues on the structure of βarr1 in the basal state (PDB ID: 1G4M). The residues with resonances exhibiting chemical shifts larger than 0.1 ppm upon binding to phosphorylated β2V2R in rHDLs bound to the full agonist are shown as red spheres. Credit: Nature Communications (2021). DOI: 10.1038/s41467-021-27482-3 Two-part binding triggers signal activation of key regulatory protein within the cell](https://scx1.b-cdn.net/csz/news/800a/2022/two-part-binding-trigg.jpg)