Graphical abstract. Credit: DOI: 10.1021/jacs.1c09508
It is attractive to convert CO2 into multicarbon hydrocarbons and oxygenates (C2+ products), but it is challenging to enhance the kinetics of carbon-carbon (C-C) coupling during CO2 reduction reaction (CO2RR).
Cu has a superior catalytic performance in C-C coupling. However, its selectivity is undermined in a neutral medium due to the acceleration of the competing hydrogen evolution reaction (HER).
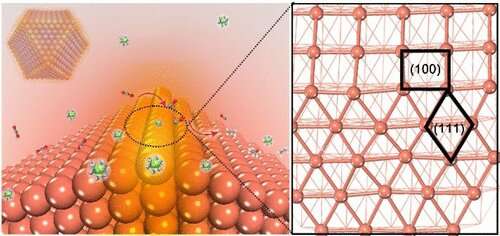
In a recent study published in Journal of the American Chemical Society, a team led by Prof. Gao Minrui from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences developed an oxide-derived (OD)-Cu catalyst with sufficient Cu(100)/Cu(111) interfaces which displayed high Faradaic efficiency (FE) at a current density of 300 mA·cm-2, and confirmed that the interfaces played a critical role in C-C coupling.
The researchers synthesized a series of OD-Cu catalysts with different facets followed by a performance test in a flow-cell with a neutral medium. They discovered that OD-Cu-III, which was composed of both Cu(100) and Cu(111) facets and had the longest Cu(100)/Cu(111) interface, exhibited superior CO2RR catalytic performance. Meanwhile, there was a linear relationship between C2+ selectivity and the length of Cu(100)/Cu(111) interface, which implied that the interface might be the active site for C-C coupling.
The following characterizations offered a more profound explanation for the superior catalytic performance of Cu(100)/Cu(111) interface. Kelvin probe force microscopy (KPFM) revealed that the interface had the lowest contact potential difference (CPD), which was indicative of faster electron transfer and enhanced CO2RR kinetics. Operando Raman spectroscopy, on the other hand, detected more surface-adsorbed CO (*CO) on step sites. Since step sites better contribute to the conversion of *CO to C2+ products, the result explained the catalysts' superior *CO dimerization ability.
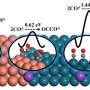
Finally, density functional theory (DFT) calculations were conducted to further understand the reaction mechanism. The result showed the lowest dimerization barrier at Cu(100)/Cu(111) interface. It implied a modified electronic structure along the interface. As a result, OD-Cu-III exhibited the best CO2RR performance.
This work determined that the Cu(100)/Cu(111) interface played a crucial role in CO2RR catalysis. It is of great significance for the utilization of CO2 to prepare multicarbon fuels.
More information: Zhi-Zheng Wu et al, Identification of Cu(100)/Cu(111) Interfaces as Superior Active Sites for CO Dimerization During CO2 Electroreduction, Journal of the American Chemical Society (2021). DOI: 10.1021/jacs.1c09508
Journal information: Journal of the American Chemical Society
Provided by Chinese Academy of Sciences