Nickel single atom and copper nanoparticles used for highly selective tandem electrocatalysis of CO2 to ethylene
The electroreduction reaction of CO2 (CO2RR) into higher-value C2+ (C≥2) products such as C2H4 provides an environmental friendly technology to realize the cyclic utilization of carbon resources, but the activity and selectivity of higher-value C2+ (C≥2) products are largely limited by the multi-electron transfer process and sluggish C-C coupling step at one single active site.
In a study published in Angewandte Chemie International Edition, Prof. Cao Rong and Prof. Huang Yuanbiao from Fujian Institute of Research on the Structure of Matter (FJIRSM) of the Chinese Academy of Sciences (CAS) developed an effective tandem catalysis strategy to improve the selectivity of CO2RR towards C2H4 by multiple distinct catalytic sites in local vicinity.
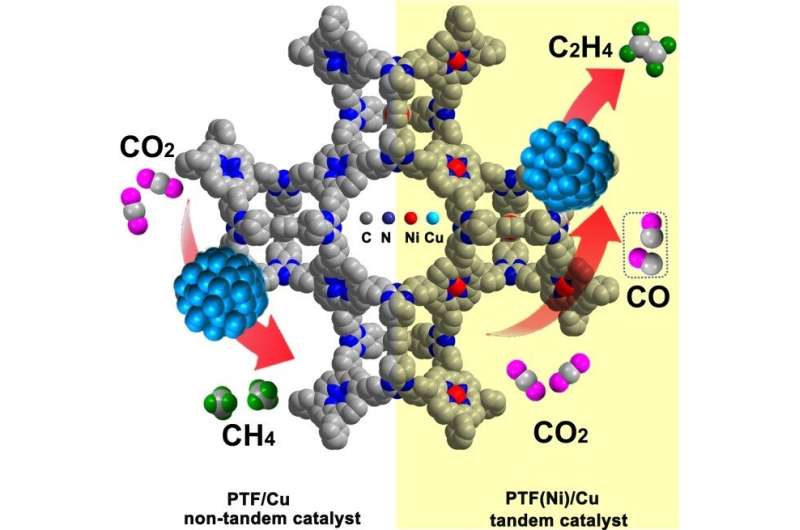
The researchers constructed an earth-abundant elements–based tandem electrocatalyst PTF(Ni)/Cu by uniformly dispersing Cu nanoparticles (NPs) on the porphyrinic triazine framework anchored with atomically isolated nickel-nitrogen sites (PTF(Ni)).
The Faradaic efficiency of C2H4 reaches 57.3 percent at -1.1 V versus the reversible hydrogen electrode (RHE) which is about six times higher than the non-tandem catalyst PTF/Cu (porphyrinic triazine framework anchored with no metal), surpassing most of the catalysts. PTF(Ni)/Cu shows good stability with continuous production of C2H4 over 11 h electrolysis as evidenced by the almost unchanged total current density and FEC2H4. The electroreduction of CO (CORR) on PTF(Ni)/Cu and PTF/Cu proved the importance of the in-situ generated CO by atomically isolated nickel-nitrogen sites, which can fast transfer to the nearby Cu NPs for the next C-C coupling reactions to form C2H4.
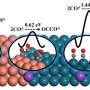
Besides, the operando ATR-FTIR and density functional theory (DFT) calculations elucidated that the high concentration of local CO on Cu sites increased *CO intermediate coverage on Cu surface and thus enhanced the C-C coupling probability, leading to the enhanced formation of C2H4, while a low CO concentration was favorable for the formation of CH4.
This study proposed a facile and general synthesis strategy to design tandem catalyst based on single-atom active sites, which can guide the subsequent design of future generations of highly efficient CO2RR catalysts to multicarbon products.
More information: Jun‐Dong Yi et al, Conductive Two‐Dimensional Phthalocyanine‐based Metal–Organic Framework Nanosheets for Efficient Electroreduction of CO2, Angewandte Chemie International Edition (2021). DOI: 10.1002/anie.202104564
Journal information: Angewandte Chemie International Edition
Provided by Chinese Academy of Sciences