Credit: University of Tsukuba
Carbonic anhydrases are essential enzymes that are present in virtually all living things; all eight classes of carbonic anhydrases that have been identified to date need a metal ion to function. But now, researchers from Japan have discovered that metal is not crucial for all carbonic anhydrases.
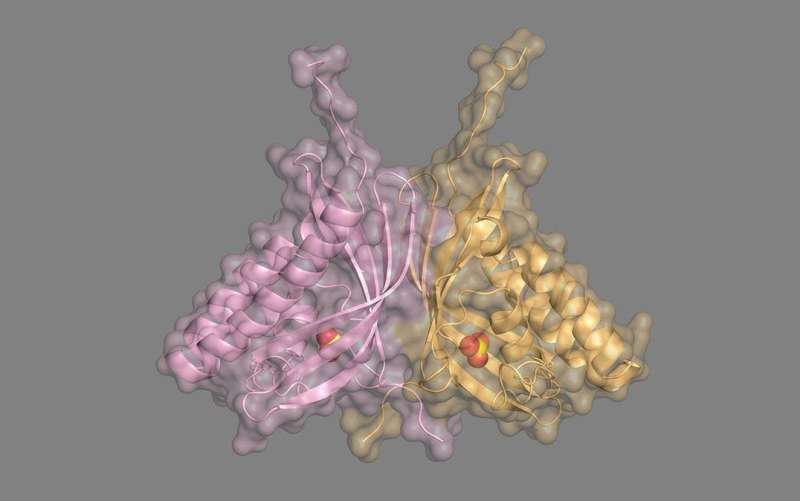
In a study published this month in BMC Biology, researchers from the Faculty of Life and Environmental Sciences at the University of Tsukuba have described two members of the COG4337 protein family that are the first known examples of carbonic anhydrase enzymes that do not require a metal ion to function.
Carbonic anhydrases catalyze the conversion of carbon dioxide (CO2) into bicarbonate (HCO3‾), and vice versa. They are central to a wide range of physiological processes, including regulation of acid-base balance, respiration, and photosynthesis, and are crucial for all carbon-based life, from bacteria to humans. All previously identified classes of carbonic anhydrase contain a metal cofactor—zinc, cadmium, cobalt, or manganese—that is essential for the activity of these enzymes, so when the researchers at the University of Tsukuba identified that the COG4337 proteins were similar to known carbonic anhydrases, they expected metal ions to be important for these new proteins too.
"Because the active site of most carbonic anhydrases contains a metal ion that facilitates interconversion between CO2 and HCO3‾, we were surprised to find that the COG4337 proteins did not require any of the eight different metals we tested, and actually were less functional in the presence of zinc," lead author Professor Yoshihisa Hirakawa says.
When the researchers made a model of COG4337 protein structure, they found that there was an active site, or pocket, that may hold on to CO2 while the enzyme converts it to HCO3‾. Interestingly, unlike other carbonic anhydrases, these novel enzymes don't seem to carry out the reverse reaction of converting HCO3‾ to CO2. In addition, the investigators saw that these COG4337 proteins tend to congregate in the plastids and mitochondria of the microalga Bigelowiella natans, which is where CO2 metabolism takes place.
"These proteins are expressed by many cyanobacteria and eukaryotic microalgae that live in environments with fairly diverse metal contents. It is possible that this enzyme evolved in an ancestral microbe to adapt to metal-poor environments, such as the open ocean," Professor Hirakawa explains.
Given the widespread expression of carbonic anhydrases in ecologically important species of microalgae, this novel type of metal-independent carbonic anhydrases may play an important role in the global carbon cycle. Understanding more about how these enzymes work could also be useful for artificial photosynthesis, an important source of renewable energy.
More information: Yoshihisa Hirakawa et al, Characterization of a novel type of carbonic anhydrase that acts without metal cofactors, BMC Biology (2021). DOI: 10.1186/s12915-021-01039-8
Journal information: BMC Biology
Provided by University of Tsukuba