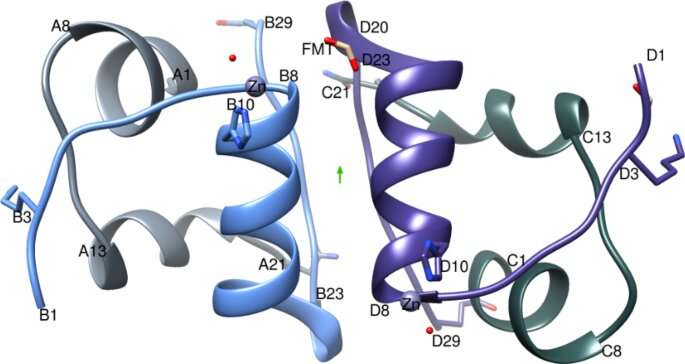
IGlu dimer with the two-fold like axis (green arrow), A-chain is coloured light grey, B-chain in light blue, C-chain in dark grey and D-chain in purple. Figure created using Chimera v1.8.1 cgl.ucsf.edu/chimera/. Credit: Scientific Reports (2021). DOI: 10.1038/s41598-021-81251-2
For the first time, scientists have come up with a precise atomic level explanation for why glulisine- a commonly used medication to treat diabetes- is faster acting than insulin.
The findings, published today in Scientific Reports, could have benefits for diabetes patients in ensuring that a more improved insulin can be developed for future treatment.
The study was carried out by experts from the Universities of Nottingham and Manchester and Imperial College London, along with the Diamond Light Source—the UK's national synchrotron science facility.
Glulisine is a synthetic rapid-acting synthetic insulin developed by Sanofi-Aventis—with a trade name of Apidra. It is used to improve blood sugar control in adults and children with diabetes.
In this new study, scientists set out to establish the exact structure of gluisine, and how this structure might affect the way in which it behaves physiologically.
The team aimed to establish, by examining the structure, what fundamental role gluisine plays in diabetes management. These findings could potentially lead to an improved synthetic insulin for patients, with fewer side effects.
Dr. Gary Adams Associate Professor and Reader in Applied Diabetes Health at the University of Nottingham, and Lead author of the study, said: "For the first time, our research provides novel, structural information on a clinically relevant synthetic insulin, glulisine, which is an important treatment for those patients presenting with diabetes.
"This information sheds light on the dissociation of glulisine and can explain its fast dissociation to dimers and monomers and thereby its function as a rapid-acting insulin. This new information may lead to a better understanding of the pharmacokinetic and pharmacodynamic behavior of glulisine and, in turn, might assist in improving its formulation and reducing side effects of this drug."
To carry out the research, the team created a perfect crystal of glulisine. The researchers then applied a combination of methods to provide a detailed insight into the structure and function of glulisine.
Dr. Hodaya Solomon, a member of the Imperial College team, and joint first author said: "The key molecular level comparisons between this crystal structure of glulisine and of previous insulin crystal structures showed that a unique position of the glutamic acid (an amino acid), not present in other fast-acting analogs, pointed inwards rather than to the outside surface. This reduces interactions with neighboring molecules and so increases preference of the more-active-for-patients dimer form, giving the experts a better understanding of the behavior of glulisine."
John Helliwell, Emeritus Professor of Chemistry at the University of Manchester, and one of the authors of the paper, said: "An unexpected finding was that the glulisine formulation is documented as a zinc-free insulin analog for its rapid absorption action. Insulin crystallography has shown that zinc is pivotal for hexamer formation. The new glulisine crystal structure showed zinc bound in the same way as in native insulin, by three histidine amino acids. This finding must mean that traces of zinc ions are present in the commercial, as supplied, formulation solution. A further optimisation for glulisine is now clear, that of finally removing the zinc."
More information: Richard B. Gillis et al. Analysis of insulin glulisine at the molecular level by X-ray crystallography and biophysical techniques, Scientific Reports (2021). DOI: 10.1038/s41598-021-81251-2
Journal information: Scientific Reports
Provided by University of Manchester