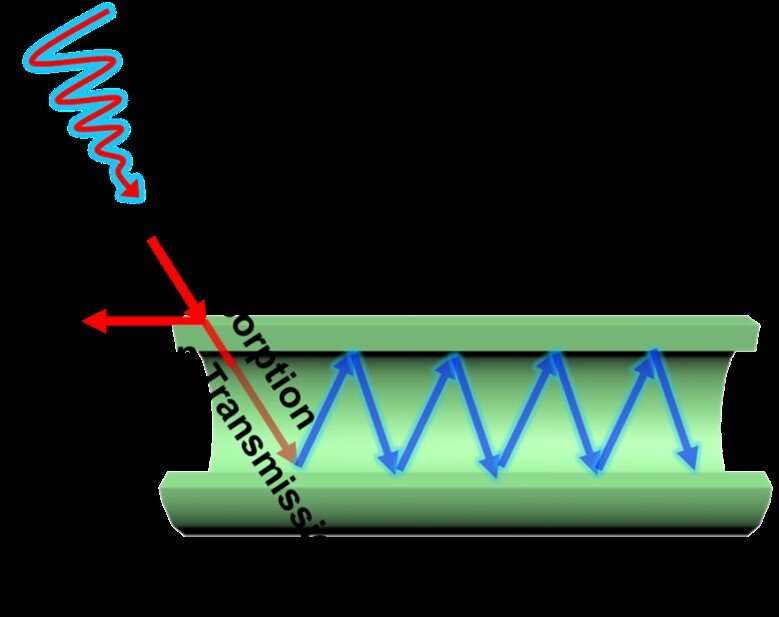
Absorbed light scatters through a titanium oxide photocatalytic tube. Credit: University of Southern Queensland 2020
Titanium dioxide (TiO2) has considerable power as a photocatalyst—a material that captures light energy to accelerate chemical reactions that are otherwise difficult to achieve. One of its most promising applications is to degrade organic (carbon-based) contaminant molecules in waste-water. In the Journal of Colloid and Interface Science researchers in China and Australia report that controlling the thickness of the walls of extremely narrow TiO2 tubes can greatly increase the photocatalytic efficiency of the material.
Light that is absorbed into the tubes is scattered along its internal surface, kicking electrons into high energy states where they can then promote the chemical reactions being catalysed. The research team have devised a method to control the thickness of the walls of the tubes, and have investigated the effect that varying thicknesses have on the efficiency of the light harvesting and catalytic behaviour.
"Our strategy to adjust the wall thickness of TiO2 nanotubes and microtubes opens a new approach to enhance the photocatalytic performance of TiO2," says corresponding author Zhi-Gang Chen at the University of Southern Queensland in Australia. Other members of the team are based at Shaanxi University of Science and Technology in China.
The nanotubes are prepared using electrospinning, a process that harnesses an electric force to pull electrically charged materials out from a solution, in this case quickly followed by solidification into the micro- and nanotubes.
A view of the photocatalytic tubes. Credit: University of Southern Queensland 2020
The work began as an attempt simply to prepare tubes with thinner walls in order to overcome a problem caused by the recombination of electrical charges when they were separated by absorbed light. "We found that in a simple one-step process we could easily adjust the wall thickness of the tubes by varying the dosage of liquid paraffin used in the solution," says Chen.
This then led to the crucial discovery that varying wall thickness had a big effect on maintaining the separation of electric charge that is crucial for the catalytic effect. "This was actually a big surprise for us," says Chen, explaining that it was proved by comparing the photocatalytic activity of five different wall thicknesses. The most effective version of the tubes was able to catalyse the degradation of two sample waste-water contaminants—dinitrophenol and rhodamine—with 99.9 percent and 97.8 percent efficiency respectively.
Chen points out that compared with other ways of making the catalytic tubes, their electrospinning method offers advantages of better control, lower costs and greater versatility in the materials it could be applied to. Similar improvements in catalysts other than TiO2 can be expected in the future.
In the meantime, TiO2 is suitable for catalysing a wide range of important reactions other than degradation of unwanted pollutant wastes. These possibilities include using the energy of the sun to power the splitting of water to generate hydrogen as a fuel, the conversion of carbon dioxide into useful products, and applications in making solar cells and electrical storage devices.
More information: Xinxin Zou et al. Tuning wall thickness of TiO2 microtubes for an enhanced photocatalytic activity with thickness-dependent charge separation efficiency, Journal of Colloid and Interface Science (2020). DOI: 10.1016/j.jcis.2020.06.081
Provided by SciencePOD