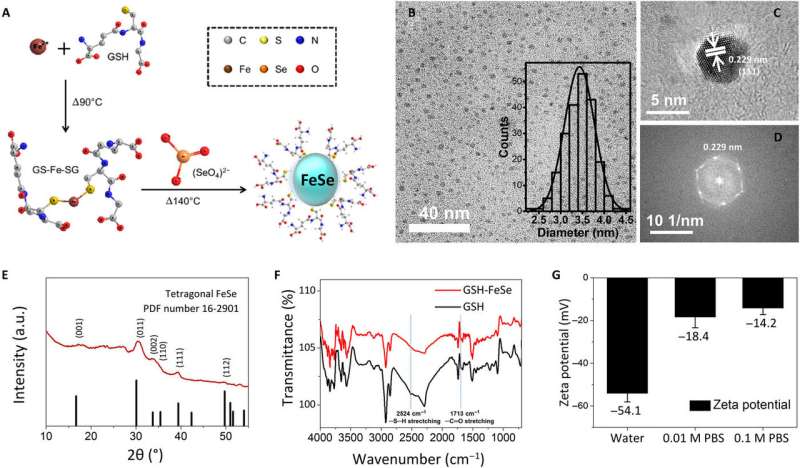
Physicochemical characterization of one-pot–synthesized water-soluble FeSe QDs. (A) Schematic illustration of the one-pot synthesis procedure for GSH-capped FeSe QDs, (B) bright-field TEM image (inset: histogram of size distribution), (C) high-resolution TEM image, (D) fast Fourier transform of high-resolution TEM image, (E) GIXRD patterns, (F) FTIR spectra, and (G) zeta potential of FeSe QDs. a.u., arbitrary units. Credit: Science Advances, doi: 10.1126/sciadv.aay0044
Photoluminescent probes with high biocompatibility, quantum yield and multiphoton absorption performance are of significant interest in biomedical imaging, expected to achieve improved penetration depth and spatial resolution. Iron selenide (FeSe) quantum dots (QDs) are reported to meet these criteria based on a new report published in Science Advances by J. Kwon and a team of researchers in the interdisciplinary departments of Chemistry, Biomaterial Science and Cogno-Mechantronics Engineering in Korea and China. Quantum dots are nanometer-scale luminescent semiconductor crystals with unique chemical and physical properties relative to their structure and composition.
The synthetic QDs in the present study can exhibit two- and three-photon excitation properties at 800- and 1800 nm wavelengths with a high quantum yield (40 percent) for second-window imaging. The materials were also biocompatible and verified by Kwon et al. when they linked poly(ethylene glycol)-conjugated QDs with human epidermal growth factor receptor 2 (HER2) antibodies for in vitro and in vivo two-photon imaging. The scientists successfully imaged the surfaces at a depth of up to 500 µm from the surface of skin using a nonlinear femtosecond laser at an excitation wavelength of 800 nm. The findings can open a new path to use biocompatible FeSe QDs for multiphoton tissue imaging during disease diagnosis.
Transition metal chalcogenides are attractive across a range of research areas in nanoscience with applications as magnetic semiconductors, superconductors, photovoltaics, electrocatalysts, sensors and quantum dots. Layered iron-based materials are promising superconductor candidates with low toxicity and cost, with unexpectedly high superconducting transition temperature. Iron chalcogenide materials can become fluorescent nanosemiconductors when their dimensions reduce to zero. These have unique optoelectronic properties relevant in biological imaging and solar power conversion. Fluorescent biomedical imaging with semiconducting nanocrystals are a promising detection technique due to high photostability and tunability of the nanocrystals during absorption and emission spectra compared to conventional organic dyes. QDs can also exhibit multiphoton-excited photoluminescence (PL) where a luminophore can simultaneously absorb more than two photons via a virtual state to emit visible light.
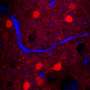
In vitro two-photon microscopic images of HER2-overexpressed MCF7 cells (positive control) after treatment with anti-HER2–PEG-QDs (20 μg ml−1). Credit: Science Advances, doi: 10.1126/sciadv.aay0044
The phenomenon can allow greater penetration depth for reduced tissue autofluorescence and scattering with benefits for in situ biomedical fluorescence imaging during cancer surgery. Scientists therefore view multiphoton microscopy (MPM) as a non-invasive, in vivo, deep-tissue imaging tool. In the present study, Kwon et al. were motivated by preceding work to synthesize biocompatible QDs with two- and three-photon luminescent properties using iron (Fe) and selenium (Se) elements. In general, the two (Fe and Se precursors) naturally occur in the human body and exhibit low toxicity in nanoparticle form. The research team tested in vitro tumor cell targeting specificity with humanized monoclonal HER2 (human epidermal growth factor receptor 2) antibody—conjugated iron selenide (FeSe) quantum dots (anti-HER2-QDs). For the in vitro experiments, they used a HER2-overexpressed MCF-7 (Michigan Cancer Foundation) xenograft model (grafts from a different donor species) of breast cancer cell line. They then conducted in vivo MPM (multiphoton microscopy) imaging within a live xenograft model of human breast tumor.
To develop the water-soluble FeSe QDs, the scientists used a one-pot synthetic strategy. They formed QDs approximating 3.4 ± 0.3 nm in size and observed them using bright-field transmission electron microscopy (TEM). Using high-resolution TEM and electron diffraction patterns of QDs, they observed the plane of tetragonal FeSe. The scientists used structural analysis with a grazing incidence X-ray diffraction spectrometer (GIXRD) and X-ray photoelectron spectroscopy to meticulously prove the morphology of FeSe QDs. Zeta potential tests showed that FeSe QDs dissolved in deionized water and in 0.01 M as well as 0.1 M phosphate-buffer (PBS) saline. When Kwon et al. monitored them using a digital camera and fluorescent microscopy after five days, the QDs did not aggregate or differ in fluorescence. The bandgap of FeSe QDs approximated 2.44 eV from the ultraviolet to visible (UV-Vis) spectrum.
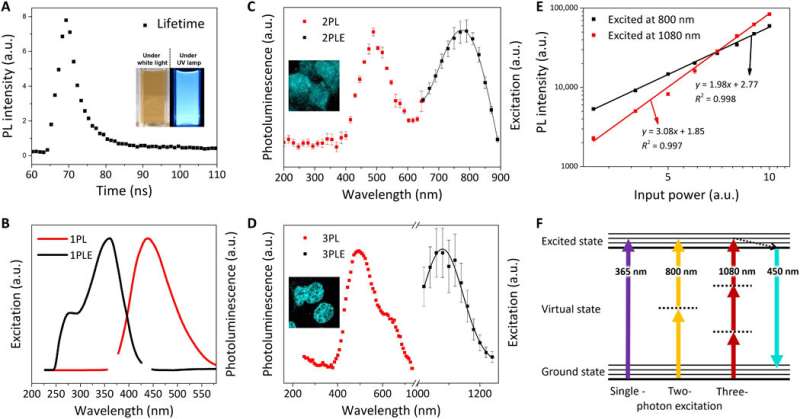
Optical characterization of FeSe QDs. (A) PL lifetime (τ) of FeSe QDs at an excitation wavelength of 380 nm; inset: digital images of FeSe dispersion under white light and UV lamp (λex = 365 nm). Normalized PL excitation (PLE) spectrum (black line) and PL spectrum (red line) at λem of 440 nm and λex of (B) 365 nm for 1PL, (C) 800 nm for 2PL, and (D) 1080 nm for 3PL. (E) Power dependence of PL intensity for 2PL (black square) and 3PL (red square). The slope of the power-dependence function is 1.98 and 3.08 for 2PL and 3PL, respectively. (F) Jablonski diagram of single-, two-, and three-photon luminescence. Credit: Science Advances, doi: 10.1126/sciadv.aay0044
Kwon et al. explored the photoluminescence (PL) properties of FeSe QDs at 25 degrees C to observe a lifetime of 3.23 nanoseconds (ns). They noted a two-photon (2PL) and three-photon (3PL) excited PL, followed by representative fluorescence microscopic images of MCF27 cells stained with FeSe QDs from 2PL and 3PL. This multiphoton excitation property is notable for bioimaging with a longer wavelength that can penetrate a maximum tissue depth with reduced phototoxicity, observed within the "golden window" during brain tissue imaging.
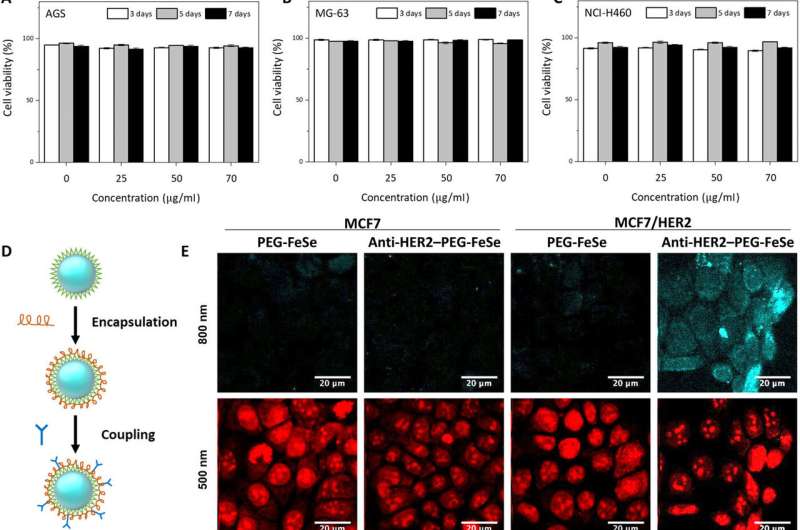
The research team first tested QD influence on cell viability before applying FeSe QDs to bioimaging experiments. They used different cell lines cultured with diverse concentrations of FeSe QDs at various culture durations and observed excellent viability by seven days, with >75 percent cell viability. Using fluorescence microscopic images of the cell cultures, Kwon et al. recorded the superior biocompatibility of FeSe QDs, where the quantum dots did not interfere with cell growth. To further minimize nonspecific binding during the assembly, the scientists encapsulated FeSe QDs with poly(ethylene glycol) (PEG) before conjugation with HER2 antibodies to develop anti-HER2-PEG-QDs.
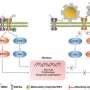
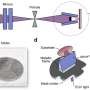
In vitro and in vivo two-photon microscopic imaging of FeSe QDs targeted to breast tumor. Flow cytometry evaluation of the viability of (A) AGS, (B) MG-63, and (C) NCI-H460 cells exposed to QDs at various concentrations (0, 25, 50, and 70 μg ml−1) for 3, 5, and 7 days. (D) Conjugation procedure to prepare anti-HER2–PEG-QDs. (E) In vitro two-photon microscopic imaging of MCF7 and HER2-overexpressed MCF7 cells (MCF7/HER2) stained with PEG-coated FeSe QDs or anti-HER2–conjugated PEG-QDs (anti-HER2–PEG-QDs, 2 μg ml−1), where the nuclei were dyed with propidium iodide, and the cell membrane and nuclei were imaged at λex of 800 and 500 nm. Laser power = 40 mW at the focal plane. (F) Comparison of the photostability of QDs and rhodamine 6G (Rh6G) in deionized water under two-photon excitation (λex = 800 nm, laser power = 50 mW), where relative PL intensity was monitored for 30 min. (G) Digital photograph of tumor xenograft for in vivo imaging. (H) MPM system. CH PMT, channel photomultiplier tube; OPO, optical parametric oscillator. (I) In vivo MPM images before and after anti-HER2–QD injection and (J) in vivo MPM images at different focal depths (450 to 500 μm). Scale bars, 20 μm. Credit: Science Advances, doi: 10.1126/sciadv.aay0044
The team tested the nonspecific uptake and selectivity of the conjugates during human breast cancer cell targeting via propidium iodide staining. The anti-HER2-PEG-QDs specifically targeted the HER2 receptors, indicating the potential to use PEGylated QDs as imaging agents in vivo. The physiologically stable molecules maintained their optical properties for seven days in serum and in a variety of buffer solutions. The FeSe QDs were highly photostable during two-photon excitation with additional properties suited for biological imaging and long-term tracking of targeted cells.
The study offered a fresh perspective for breast cancer diagnosis. Breast cancer is the second-highest cause of cancer death for women, with significant recurrence rates, where minimally invasive surgery aided with sensing and imaging techniques are crucial to identify the disease. The research team established an in vivo MPM (multiphoton microscopy) imaging method with intravenous injection of anti-HER2-PEG-QDs in an MCF xenograft animal model. They then established a subcutaneous xenograft mouse model of breast cancer by injecting MCF7 cells and MCF/HER2 cells into the flank of mice. After four weeks, when the tumor volume reached 200 mm3, the scientists injected 100 µL of anti-HER2-PEG-QDs and observed the FeSe QDs as a magenta signal. They then obtained 2PL signals at different depths across the tumor area at regular intervals. The second harmonic generation (SHG) signal appeared blue to represent collagen in the superficial area and the scientists distinguished the PL signal from QDs near the breast cancer cells.
In vivo two-photon microscopic images (Z-scan) of cancer part were obtained 30 min after intravenous tail vein injection of anti-HER2–PEG-QDs by moving the excitation focal plane from 450 to 525 μm from the skin in 5-μm steps (λex = 800 nm, power = 100 mW). Credit: Science Advances, doi: 10.1126/sciadv.aay0044
In this way, J. Kwon and colleagues synthesized biocompatible FeSe QDs with strong cell viability at increased QD concentrations. The team used QDs during two- and three-photon fluorescence imaging and with multiphoton imaging at a depth of up to 500 µm to monitor tumor cells with a nonlinear femtosecond laser within live animals in vivo. The combined biocompatible FeSe QDs and multiphoton imaging can open a new method to realize noninvasive in situ bioimaging within live subjects.
More information: J. Kwon et al. FeSe quantum dots for in vivo multiphoton biomedical imaging, Science Advances (2019). DOI: 10.1126/sciadv.aay0044
Wei Li et al. Phase separation and magnetic order in K-doped iron selenide superconductor, Nature Physics (2011). DOI: 10.1038/nphys2155
Xingyong Wu et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots, Nature Biotechnology (2003). DOI: 10.1038/nbt764
Journal information: Science Advances , Nature Physics , Nature Biotechnology
© 2019 Science X Network